Session Information
Session Type: Poster Session A
Session Time: 10:30AM-12:30PM
Background/Purpose: Large granular lymphocyte (LGL) leukemia is a rare hematologic malignancy characterized by clonal expansion of cytotoxic T-cells and frequent somatic activating STAT3 mutations. A major hallmark of all LGL leukemia is increased STAT3 activation. About 50% of patients have somatic activating mutations in STAT3, the most common being H640F and D661Y. Additionally, STAT3 mutations correlate with numerous clinical features in LGL leukemia. LGL leukemia patients with STAT3 mutations are more likely to respond to methotrexate treatment, and tend to have low neutrophil counts. Approximately one third of LGL leukemia patients also suffer from RA, and those with STAT3 mutations are more likely to exhibit concomitant RA. Those patients with both diseases also tend to have increased positivity for serological markers like RF- and anti-CCP antibodies than patients with either disease alone. Based on the disease overlap between LGL leukemia and rheumatoid arthritis (RA) and a putative role for CD8+ T-cells in RA, we hypothesized that STAT3 mutations may be detected in RA patient CD8+ T cells and correlate with clinical characteristics.
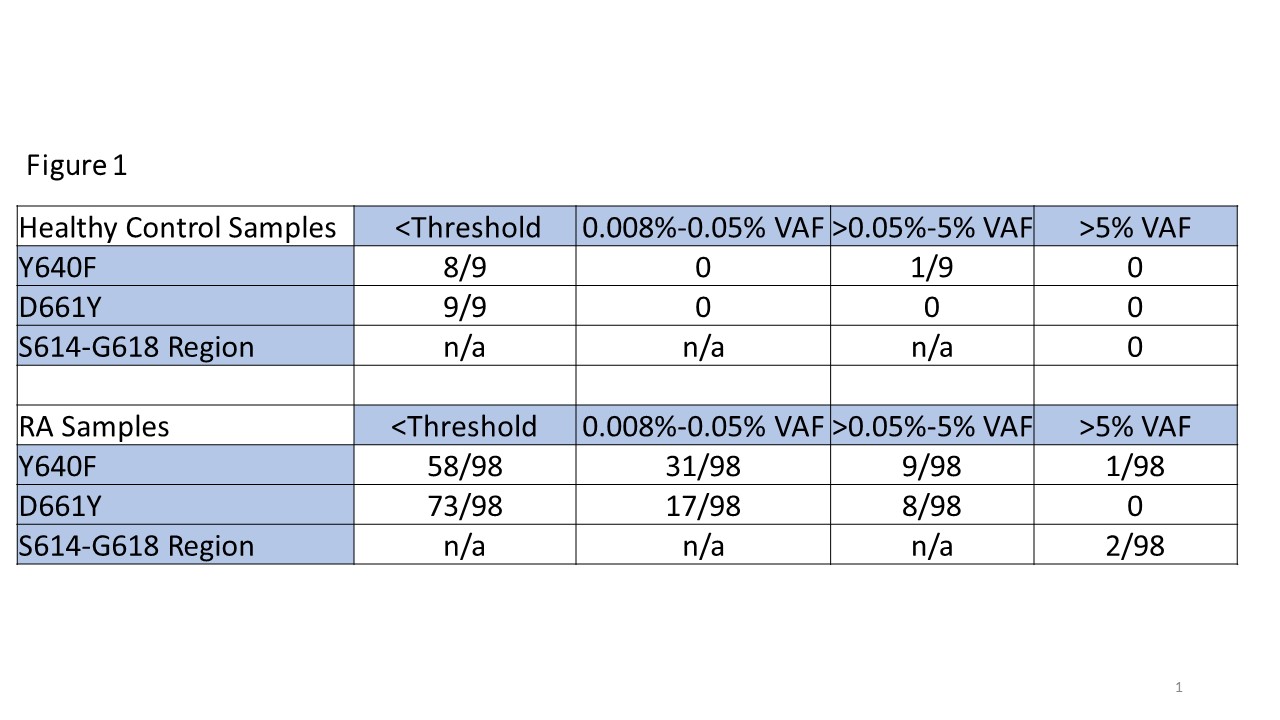
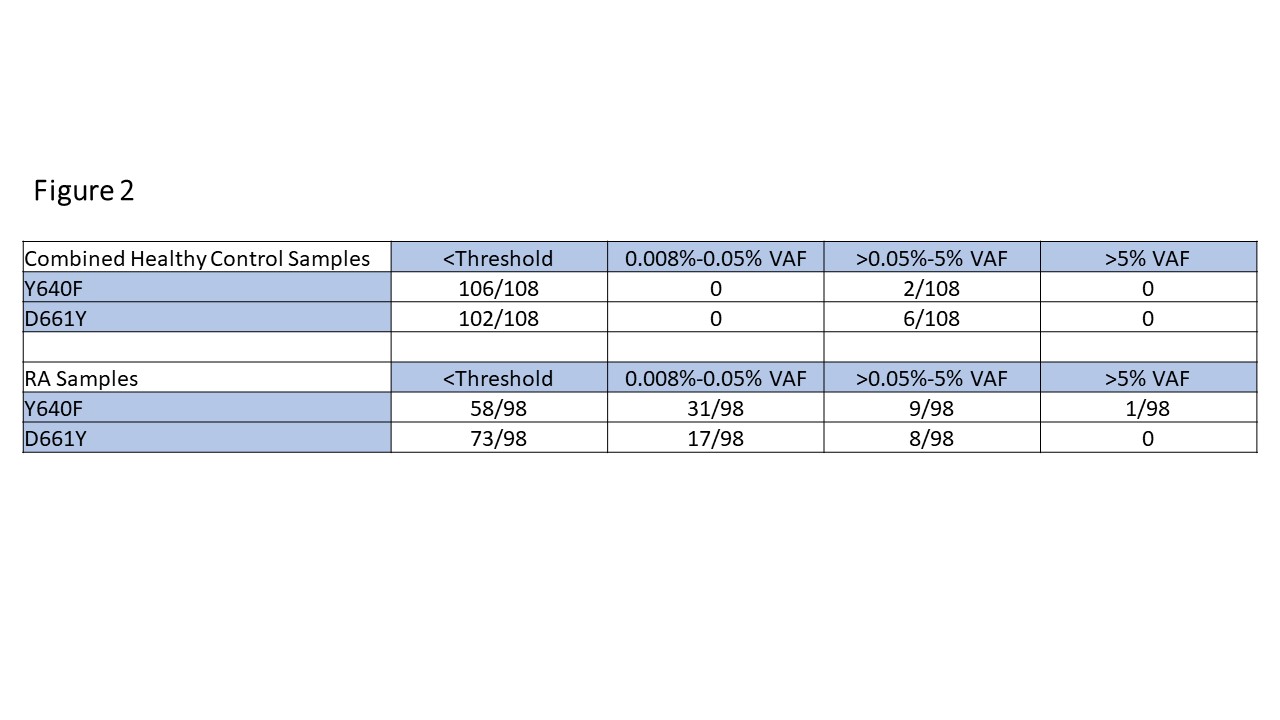
Methods: Blood samples, clinical parameters, and demographics were collected from 98 RA patients and 9 healthy controls (HCs). CD8+ cell DNA was isolated and analyzed via droplet digital (dd)PCR to detect STAT3 mutations common in LGL leukemia: Y640F, D661Y, as well as identifying mutations in the S614 to G618 region by dropout assay, and the resulting variant allele frequencies (VAF) were recorded (Figure 1). Additional STAT3 data from 99 HCs from a public dataset supplemented our 9 HCs. RA patients were then split into STAT3 mutant (VAF >0.05%) and WT groups, and analysis was performed to determine clinical correlations with mutational status (Figure 2).
Results: RA patients had significantly increased levels of STAT3 mutations compared to controls (Y640F p=0.0005, D661Y p=0.0005). The majority of this difference stemmed from low level mutations (0.008-0.05% VAF) detected in the RA population (31/98 Y640F, 17/98 D661Y) vs. HCs (0/108 Y640F, 0/108 D661Y) (Figure 2). In addition, 3/98 RA samples had a STAT3 mutation at a VAF >5% compared to 0/108 controls (Figure 1).
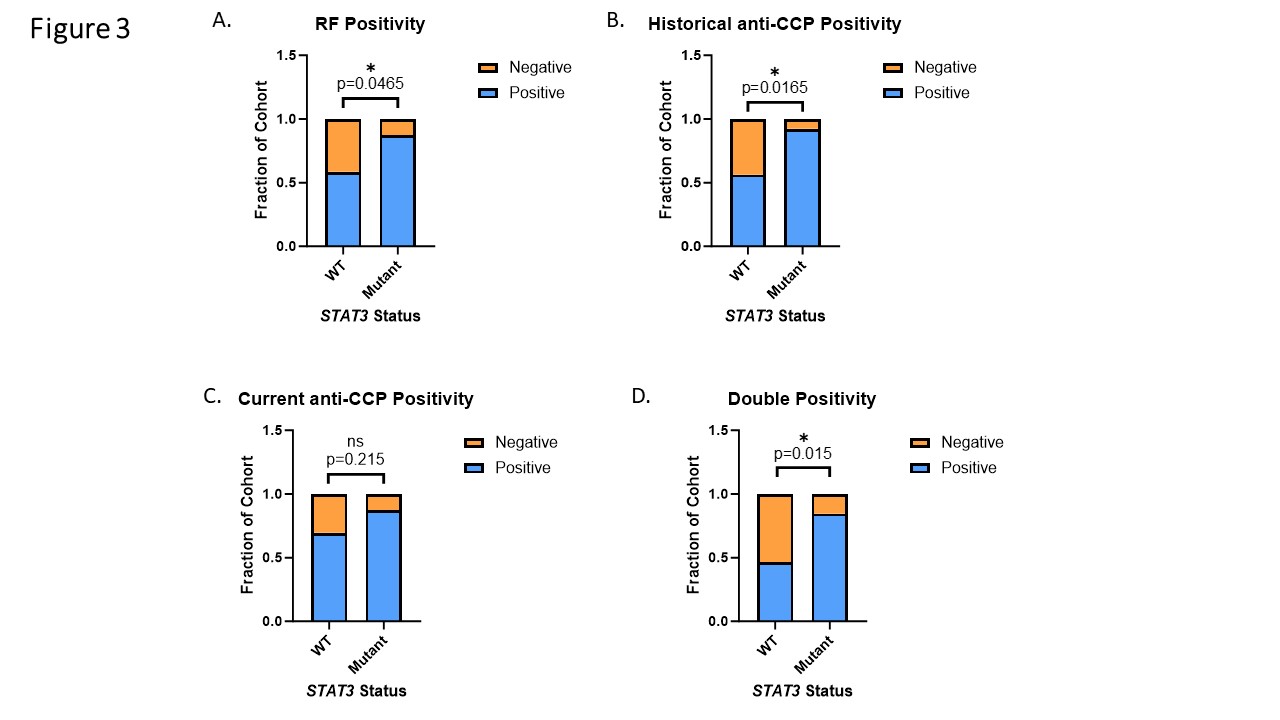
Serological markers, RF and anti-CCP positivity, were more frequently positive in RA patients with STAT3 mutation (88% RF, p = 0.047; 92% anti-CCP, p = 0.017) relative to those without (59% RF, 56% anti-CCP). D) RA patients with STAT3 mutations exhibited more double positivity for both RF and anti-CCP than WT patients (11/13, 85% vs 37/80, 46% p = 0.015 Pearson’s Chi Square) (Figure 3).
Conclusion: STAT3 activating mutations were detected in CD8+ cells and associated with seropositivity. Thus, STAT3 activating mutations may play a role in the etiology and clinical presentation in a subset of RA. Notably, methotrexate is first line therapy for LGL leukemia and its common use in treatment of RA may influence the true prevalence of these cells in RA patients. Evaluation of RA patients at various points in the course of their disease course will provide further understanding of the role of STAT3 mutations in RA.
To cite this abstract in AMA style:
moosic K, Olson T, Freijat M, khalique s, Hamele C, Shemo B, Boodoo J, Baker W, Khurana G, Schmachtenberg M, Duffy T, Ratan A, Darrah E, Andrade F, Jones M, Olson K, Feith D, Loughran T, Kimpel D. Activating STAT3 Mutations in CD8+ T Cells Correlate to Serological Positivity in Rheumatoid Arthritis [abstract]. Arthritis Rheumatol. 2024; 76 (suppl 9). https://acrabstracts.org/abstract/activating-stat3-mutations-in-cd8-t-cells-correlate-to-serological-positivity-in-rheumatoid-arthritis/. Accessed .« Back to ACR Convergence 2024
ACR Meeting Abstracts - https://acrabstracts.org/abstract/activating-stat3-mutations-in-cd8-t-cells-correlate-to-serological-positivity-in-rheumatoid-arthritis/