Session Information
Session Type: Abstract Session
Session Time: 11:15AM-11:30AM
Background/Purpose: Dysregulation in the protein C anticoagulant pathway, more specifically acquired resistance to activated protein C (APCr) and antibodies against protein C (anti-PC), has been proposed as a potential contributing mechanism in the pathophysiology of antiphospholipid syndrome (APS). However, prevalence, clinical significance, and predictive value have not been established.To determine the prevalence of APCr and anti-PC, their associations with antiphospholipid antibody (aPL) profiles and clinical phenotypes, and their predictive value for new thrombotic events.
Methods: Patients with aPL with/without APS classification were identified from the prospective APS ACTION registry (n=283) and University College London Hospital database (n=87). Fifty-one healthy controls were also included. Patients (n=370) were subcategorised by clinical phenotype: aPL only (n=77), pregnancy morbidity (PM) only (n=43), venous thromboembolism (VTE) (+/-PM) (n=132), arterial thrombosis (AT) (+/-PM) (n=87), or VTE+AT (+/-PM) (n=31). Based on baseline samples of different subgroups, APCr was determined using thrombin generation with recombinant human APC (rhAPC) or Protac (for endogenous protein C activation). Anti-PC and avidity were detected by in-house ELISA. In a subgroup analysis, based on prospective APS ACTION data, the incidence of new thrombotic events was compared in patients with and without APCr and anti-PC.
Results:
Results: All patient subgroups had markedly greater APCr than healthy controls (p < 0.001), most prominent in ‘higher risk’ patients, i.e., those with VTE+AT or triple aPL-positivity (Figure 1). Forty-two percent (156/370) of patients were positive for anti-PC with the highest prevalence in the PM, VTE and VTE+AT cohorts: 53%, 47%, and 45%, respectively, and lowest prevalence in aPL only patients (34%) (Figure 2A). The VTE+AT group had significantly higher prevalence of high avidity anti-PC (79%) compared to aPL only patients (38%) (p < 0.05, Figure 2B). Most patients with APCr (69.5% [141/370]) had detectable anti-PC. There was a strong association between presence of APCr and anti-PC (Figure 2D) especially in the VTE+AT and the triple aPL positive patient groups. During the follow-up (median 8.3 years) of APS ACTION patients, 25/283 (8.8%) patients had new thrombosis; there was no difference in the number of new thrombotic events in patients with or without APCr or anti-PC (p=0.60). Non-anticoagulated patients with APCr had a higher number of new thrombotic events compared to those without APCr, although this did not reach statistical significance (HR 4.5, 95% CI 0.8-25.9, p=0.14).
Conclusion: Acquired resistance to activated protein C is highly prevalent in persistently aPL-positive patients with the highest prevalence in ‘higher risk’ APS patients. The lack of an association with thrombosis in these patients may reflect the effectiveness of anticoagulation. The association between greater APCr and thrombosis in non-anticoagulated patients merits exploration as to whether this may provide a basis to guide consideration of initiation of prophylactic antithrombotic treatment in this subset of patients.
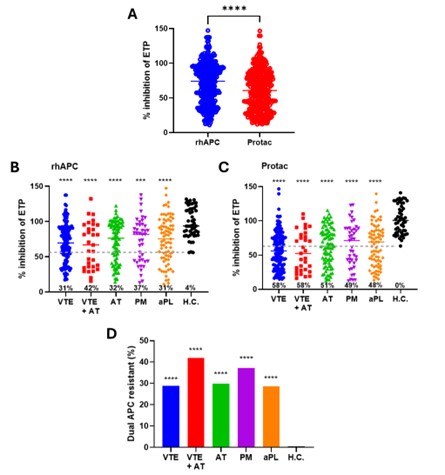 Activated protein C (APC) resistance in the different aPL cohorts. Thrombin generation was measured using platelet-poor plasma (PPP)in patients with aPL (n=370) with or without antiphospholipid syndrome (APS) and healthy controls (H.C., n=51); A: Paired comparison of % inhibition of ETP by rhAPC and Protac in the patient cohort (n=370). Patients were subcategorised into clinical manifestations of venous thromboembolism (VTE, n=132), arterial thromboembolism (AT, n=87), VTE + AT (n=31), pregnancy morbidity (PM, n=43), carriers of aPL (aPL, n=77) and compared to H.C. (n=51) with rhAPC (B) and Protac (C). Grey dotted lines (56% for rhAPC, 63% for Protac) indicate cut-offs established in HC. (56% for rhAPC, 63% for Protac) are considered APC resistant, with percentages shown. The proportion of each clinical subcategory that were resistant to both rhAPC and Protac are shown (D), compared to H.C. ***p < 0.001, ****p < 0.0001
Activated protein C (APC) resistance in the different aPL cohorts. Thrombin generation was measured using platelet-poor plasma (PPP)in patients with aPL (n=370) with or without antiphospholipid syndrome (APS) and healthy controls (H.C., n=51); A: Paired comparison of % inhibition of ETP by rhAPC and Protac in the patient cohort (n=370). Patients were subcategorised into clinical manifestations of venous thromboembolism (VTE, n=132), arterial thromboembolism (AT, n=87), VTE + AT (n=31), pregnancy morbidity (PM, n=43), carriers of aPL (aPL, n=77) and compared to H.C. (n=51) with rhAPC (B) and Protac (C). Grey dotted lines (56% for rhAPC, 63% for Protac) indicate cut-offs established in HC. (56% for rhAPC, 63% for Protac) are considered APC resistant, with percentages shown. The proportion of each clinical subcategory that were resistant to both rhAPC and Protac are shown (D), compared to H.C. ***p < 0.001, ****p < 0.0001
.jpg) Figure 2. APC resistance and anti-protein C antibodies. Citrated plasma from APS patients with VTE, n=132, ATE, n=87, VTE + ATE (n=31), PM, n=43, aPL only, n=77 and H.C., n=51 were diluted 1:25 and run on an in-house protein C ELISA to detect anti-protein C (anti-PC) antibodies. Antibody titres of subjects in each group are shown, compared to H.C. (A): Values on or above the grey dotted line (36U/mL) are considered positive for anti-PC antibody, with percentage of each group positive for antibody shown. The proportion of the antibodies detected (VTE n=61, VTE + AT n=14, AT n=31, PM n=23, aPL n=26) that were high avidity were compared to the aPL only cohort. (B): The antibody titres of all high avidity (n=88) and low avidity (n=66) anti-PC were compared. (C): High avidity anti-PC were significantly at higher titres compared to low avidity anti-PC. (D) The proportion of patients with no APC resistance (n=167), recombinant human APC (rhAPC) resistance (n=122), Protac resistance (n=196) and resistance to both rhAPC and Protac (n=115) with anti-protein C antibodies. (E): The proportion of anti-protein C antibodies that were high avidity in each of the APC resistance groups. *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001, ns – not significant, resis. – resistance
Figure 2. APC resistance and anti-protein C antibodies. Citrated plasma from APS patients with VTE, n=132, ATE, n=87, VTE + ATE (n=31), PM, n=43, aPL only, n=77 and H.C., n=51 were diluted 1:25 and run on an in-house protein C ELISA to detect anti-protein C (anti-PC) antibodies. Antibody titres of subjects in each group are shown, compared to H.C. (A): Values on or above the grey dotted line (36U/mL) are considered positive for anti-PC antibody, with percentage of each group positive for antibody shown. The proportion of the antibodies detected (VTE n=61, VTE + AT n=14, AT n=31, PM n=23, aPL n=26) that were high avidity were compared to the aPL only cohort. (B): The antibody titres of all high avidity (n=88) and low avidity (n=66) anti-PC were compared. (C): High avidity anti-PC were significantly at higher titres compared to low avidity anti-PC. (D) The proportion of patients with no APC resistance (n=167), recombinant human APC (rhAPC) resistance (n=122), Protac resistance (n=196) and resistance to both rhAPC and Protac (n=115) with anti-protein C antibodies. (E): The proportion of anti-protein C antibodies that were high avidity in each of the APC resistance groups. *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001, ns – not significant, resis. – resistance
To cite this abstract in AMA style:
Efthymiou M, Tohidi-Esfahani I, Venturelli V, Tektonidou M, Pengo V, Parades-Ruiz D, Branch W, Gerosa M, Nalli C, Rodriguez Almaraz E, Petri M, Cervera R, Amengual O, Andrade D, Willis R, Bertolaccini M, Erkan D, Cohen H. Activated protein C resistance and protein C antibodies in the antiphospholipid syndrome alliance for clinical trials and international networking (APS ACTION) clinical database and repository [abstract]. Arthritis Rheumatol. 2025; 77 (suppl 9). https://acrabstracts.org/abstract/activated-protein-c-resistance-and-protein-c-antibodies-in-the-antiphospholipid-syndrome-alliance-for-clinical-trials-and-international-networking-aps-action-clinical-database-and-repository/. Accessed .« Back to ACR Convergence 2025
ACR Meeting Abstracts - https://acrabstracts.org/abstract/activated-protein-c-resistance-and-protein-c-antibodies-in-the-antiphospholipid-syndrome-alliance-for-clinical-trials-and-international-networking-aps-action-clinical-database-and-repository/
