Session Information
Session Type: Poster Session A
Session Time: 9:00AM-11:00AM
Background/Purpose: Juvenile idiopathic arthritis (JIA) is the most common chronic inflammatory rheumatic disease of childhood and most children require long-term treatment. Adverse events (AE) during treatment may negatively influence disease control and quality of life, and may result in permanent harm.
In this study, we aim to describe the frequency, seriousness and consequences of physician reported actionable AE in an inception cohort of children with JIA, the Canadian Alliance of Pediatric Rheumatology Investigators (CAPRI) JIA Registry.
Methods: For this study, registry data were extracted on December 18, 2021. Recruitment into the CAPRI JIA registry began in February 2017 to prospectively collect information on children within 3 months of JIA diagnosis at 16 participating sites. Core data were collected at every clinic visit including information on actionable AE since previous visit reported by clinicians. An actionable AE was defined as any untoward medical occurrence that requires additional medical visits, investigations, treatment, or a change in arthritis medications, irrespective of its cause. A serious AE was defined as an event that results in death, is life-threatening, requires hospitalization, or results in a significant disability or a congenital anomaly (or requires medical intervention to prevent these).
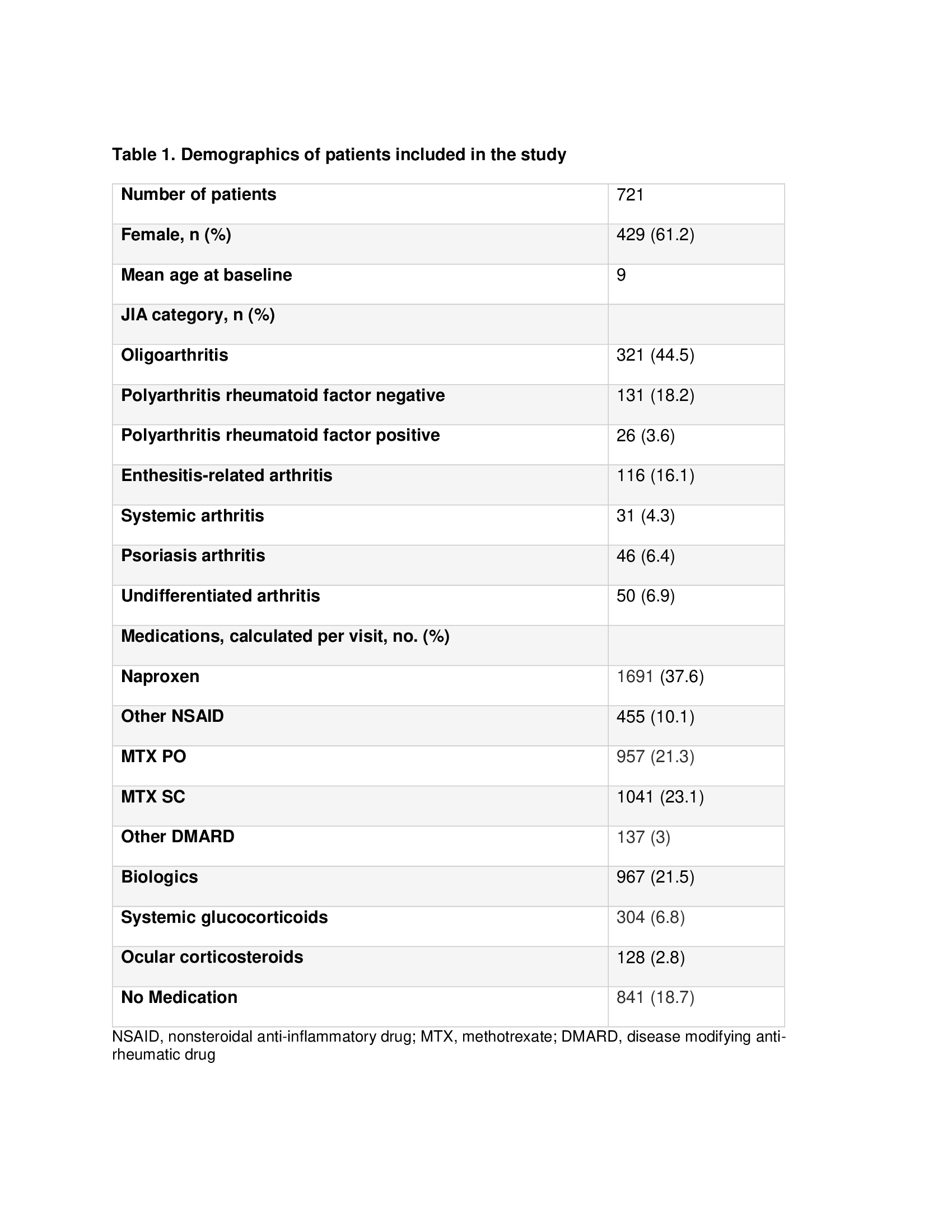
Results: Patients included in the study were comparable to Western JIA inception cohorts (Table 1). Actionable AE were reported in 261 out of 721 patients (36.2%) at 478 of 4503 visits (10.6%), during a total observation period of 1,207 patient years. The AE rate was 39 per 100 patient years. The serious AE rate was 2.9 events per 100 patient years. The probability that a patient newly diagnosed with JIA will develop an AE during the first year after diagnosis was 34% (95% CI 30-38, Kaplan-Meier estimate). Interestingly, for 67 (25.7%) patients an AE was reported at the study enrolment visit. Most patients (60%) had only 1 visit with an AE but some had multiple visits (range 1-8). The most frequent reported AE were gastrointestinal, primarily nausea and vomiting (n=217, 45.4% of all AE) and abdominal pain (n=51, 10.7%). There were 34 serious AE in 27 patients. The most frequent serious AE were infections with hospitalization (n=9, 0.75 per 100 patient-years), followed by eye surgery (n=5) and gastrointestinal bleed (n=4). There were no reported deaths, malignancies, demyelinating diseases or tuberculosis. More than half (53.8%) of the visits with a reported AE were in patients receiving non-steroidal anti-inflammatory drug (NSAID) either alone or in combination with other medications, most frequently if the combination was with methotrexate. The most common action taken by the clinician reporting an AE was additional treatment (n= 171, 35.8%), followed by stopping medication (n= 122, 25.5%), and by changing dose or route of medication (n= 93, 19.5%).
Conclusion: In our cohort, actionable AE were reported in over a third of JIA patients, but serious AE were rare. Most AE were gastrointestinal and managed with additional treatment. Increasing awareness to these actionable AE is important to improve health outcomes of patients with JIA.
To cite this abstract in AMA style:
Alnuaimi B, Batthish M, Berard R, Boire G, Campillo S, Chhabra A, Couture J, Dancey P, Feldman B, Gerschman T, Herrington J, Houghton K, Huber A, LeBlanc C, Lim L, Proulx-Gauthier J, Schmeling H, Scuccimarri R, Tucker L, Guzman J, Chédeville G, Registry Investigators F. Actionable Adverse Events in a Real-Practice Cohort of Children with Juvenile Idiopathic Arthritis. Results from the CAPRI Registry [abstract]. Arthritis Rheumatol. 2023; 75 (suppl 9). https://acrabstracts.org/abstract/actionable-adverse-events-in-a-real-practice-cohort-of-children-with-juvenile-idiopathic-arthritis-results-from-the-capri-registry/. Accessed .« Back to ACR Convergence 2023
ACR Meeting Abstracts - https://acrabstracts.org/abstract/actionable-adverse-events-in-a-real-practice-cohort-of-children-with-juvenile-idiopathic-arthritis-results-from-the-capri-registry/