Session Information
Date: Saturday, November 7, 2020
Title: Spondyloarthritis Including Psoriatic Arthritis – Treatment Poster II
Session Type: Poster Session B
Session Time: 9:00AM-11:00AM
Background/Purpose: Assessment of SpondyloArthritis international Society (ASAS) response criteria and AS Disease Activity Score (ASDAS) are both commonly used, rigorous composite indices consisting of components with relevance to patients. Clinically meaningful thresholds for these measures have been defined to reflect partial remission (PR), inactive disease (ID), and low disease activity (LDA). The objective of this analysis was to study the association of ASAS PR and ordinal ASDAS disease categories (including ASDAS ID, which is the most stringent category of this composite score) in upadacitinib (UPA)-treated patients with AS.
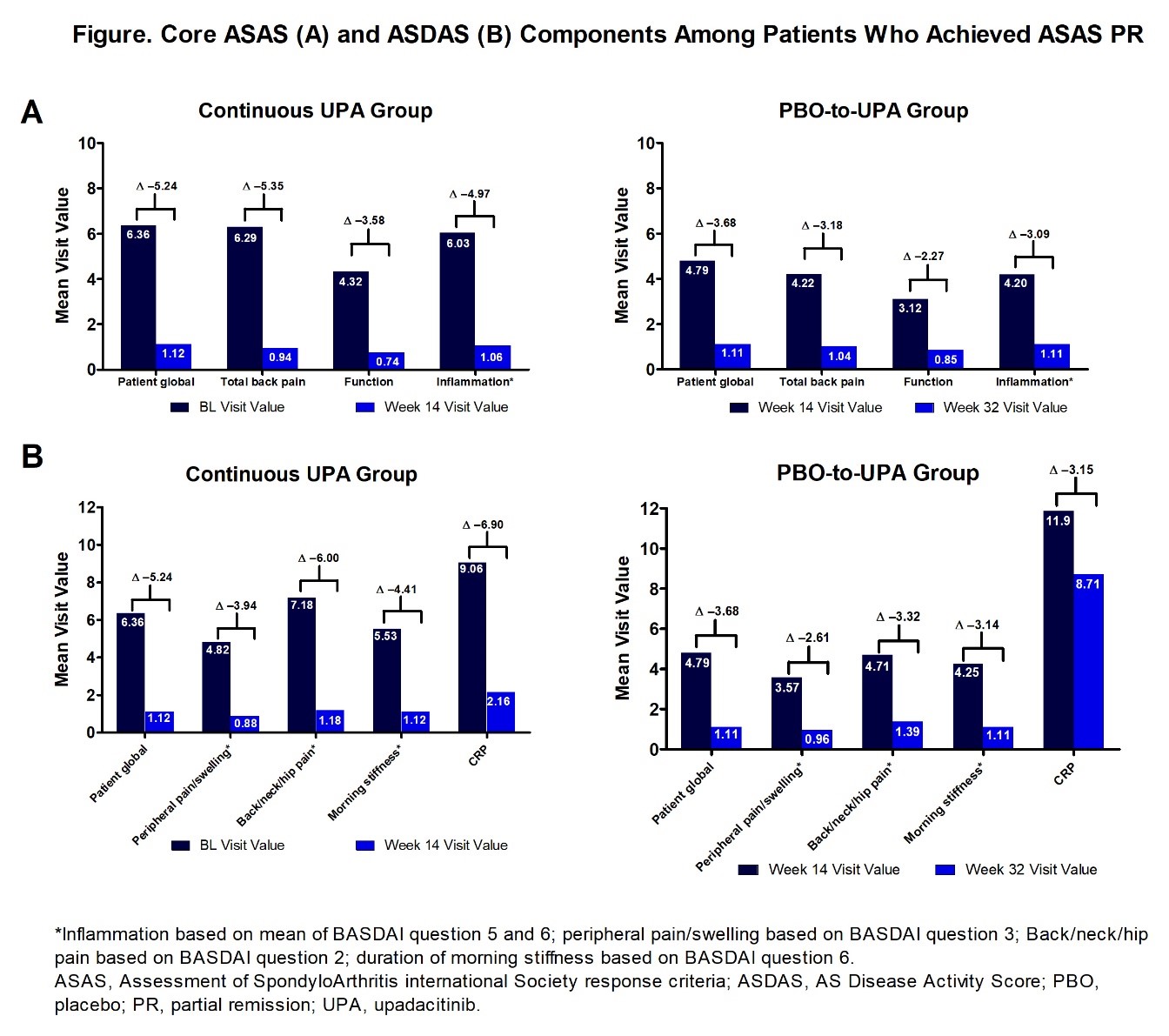
Methods: In the SELECT-AXIS 1 (NCT03178487) study, biologic DMARD naïve-patients (pts; ≥18 y) with active AS and intolerance/contraindication or inadequate response to ≥2 NSAIDs were randomized 1:1 to UPA 15 mg once daily (QD) or placebo (PBO).1 At wk 14, pts entered an open-label extension (OLE) of UPA 15 mg QD; pts randomized to PBO were switched to UPA. This post hoc analysis assessed the responsiveness of individual ASAS and ASDAS core components among pts who achieved ASAS PR. The association of ASAS PR with achievement of ASDAS ID (ASDAS < 1.3), ASDAS LDA (ASDAS < 2.1 but ≥1.3) or ASDAS high disease activity (HDA)/very HDA (VHDA) (ASDAS ≥2.1 for HDA/VHDA) was also assessed by measures including Youden index, distance to perfect point, and sensitivity/specificity equality. These evaluations were performed in pts randomized to UPA from baseline (BL; continuous UPA, assessed at wk 14) and those who were randomized to PBO and switched to UPA upon entry in the OLE (PBO to UPA; re-baselined at wk 14 and assessed at wk 32, representing 18 wks of UPA exposure).
Results: At wk 14, for the continuous UPA group, 16 pts (19%) achieved ASAS PR. At wk 32, following 18 wks of UPA exposure for the PBO-to-UPA group, 28 pts (33%) achieved ASAS PR. Among both groups (continuous UPA and PBO-to-UPA), improvements were seen across all core components (Figure). Of the 44 total pts who achieved ASAS PR, 91% achieved either ASDAS ID or LDA. The majority of patients who achieved ASAS PR achieved ASDAS ID in the continuous UPA and PBO-to-UPA groups: 11/16 (69%) and 16/28 (57%), respectively. For the continuous UPA group, the remaining 5 pts who achieved ASAS PR also achieved ASDAS LDA (Table). ASAS PR was associated with ASDAS categories in the following manner: the highest rate of ASAS PR was achieved for ASDAS ID followed by ASDAS LDA followed by ASDAS HDA/VHDA. The cutoff of 1.3 (the upper threshold for ASDAS ID) was a better discrimination threshold for ASAS PR than the cutoff of 2.1 (the upper threshold for ASDAS LDA).
Conclusion: Nineteen percent of pts receiving UPA from BL achieved ASAS PR after 14 wks of treatment, with similar results seen in pts who were originally randomized to PBO and switched to UPA at wk 14. A consistent improvement was seen across all core components of ASAS among those who achieved ASAS PR with UPA treatment. The achievement of ASAS PR was most closely associated with the achievement of ASDAS ID, providing further clarity on the reduction of disease activity in AS pts treated with UPA.
References:
- van der Heijde, et al. Lancet. 2019;394(10214):2108-2117.
To cite this abstract in AMA style:
Deodhar A, Östör A, Maniccia A, Ganz F, Gao T, Chu A, Poddubnyy D. Achievement of Partial Remission and Inactive Disease in Upadacitinib-Treated Patients with Ankylosing Spondylitis [abstract]. Arthritis Rheumatol. 2020; 72 (suppl 10). https://acrabstracts.org/abstract/achievement-of-partial-remission-and-inactive-disease-in-upadacitinib-treated-patients-with-ankylosing-spondylitis/. Accessed .« Back to ACR Convergence 2020
ACR Meeting Abstracts - https://acrabstracts.org/abstract/achievement-of-partial-remission-and-inactive-disease-in-upadacitinib-treated-patients-with-ankylosing-spondylitis/