Session Information
Date: Sunday, November 13, 2022
Title: Pediatric Rheumatology – Clinical Poster II: Connective Tissue Disease
Session Type: Poster Session C
Session Time: 1:00PM-3:00PM
Background/Purpose: This open-label 24-week study was conducted to evaluate the safety and efficacy of abatacept in patients with refractory juvenile dermatomyositis (JDM).
Methods: Ten patients of ≥7 years of age with refractory JDM were evaluated every six weeks over a 24-week period examining safety and treatment responses. The primary endpoint was the Definition of Improvement (DOI) based on the International Myositis Assessment and Clinical Studies Group (IMACS). Secondary endpoints included minimal, moderate, and major improvement from the Pediatric Rheumatology International Trials Organization (PRINTO), as well as ACR-EULAR response criteria for JDM using aggregate Total Improvement Scores (TIS). Safety was assessed by NCI CTC. MRI of the pelvis and thighs with axial STIR and T1 images were assessed. Interferon gene score (IFNGS) was performed on RNA from whole blood by NanoString Technologies (Seattle, WA) and cytokines/chemokines panel by Luminex assay (ThermoFisher Scientific, Waltham, MA). Differences were evaluated by paired t-test and Wilcoxon signed rank test. Spearman correlations (rs) were assessed.
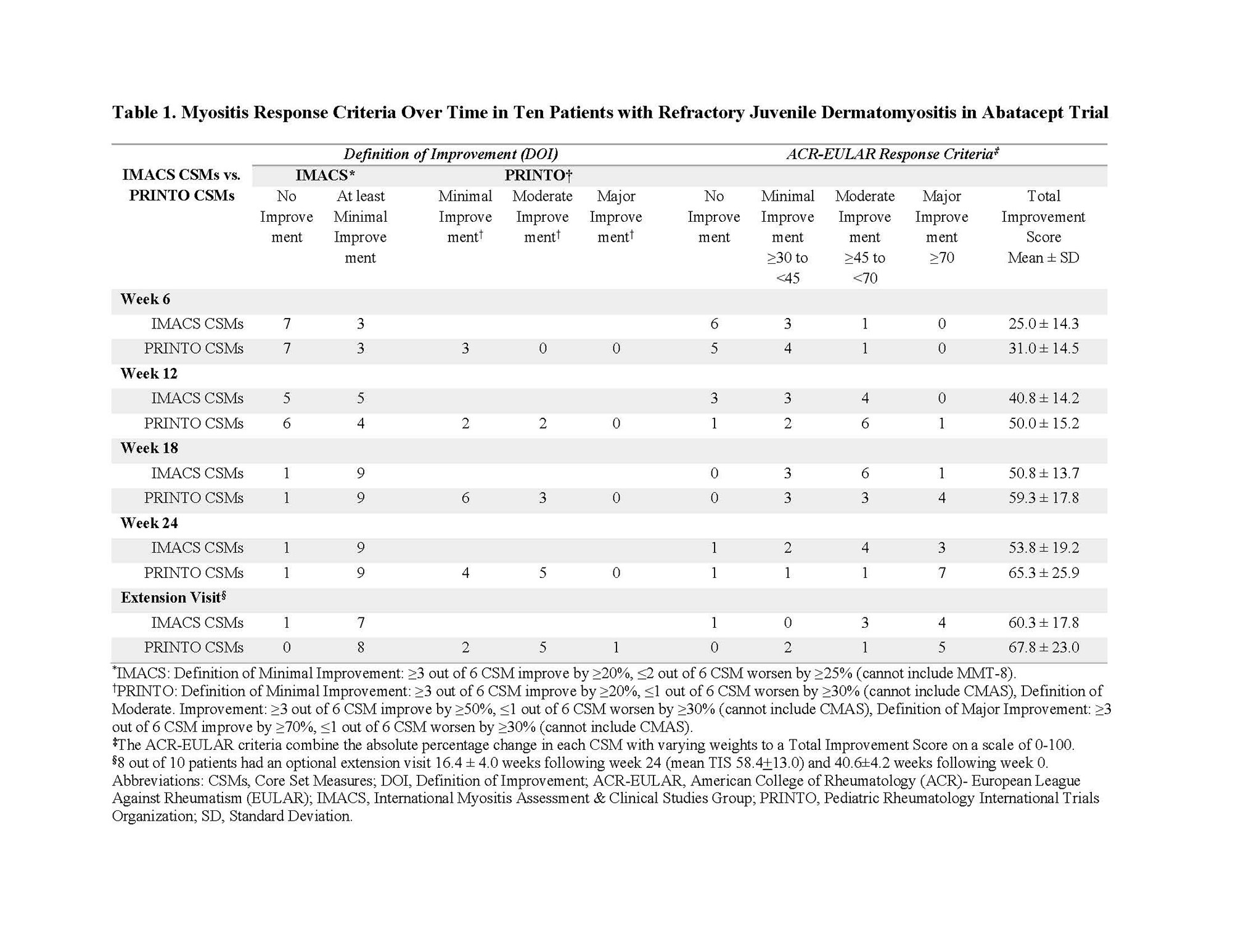
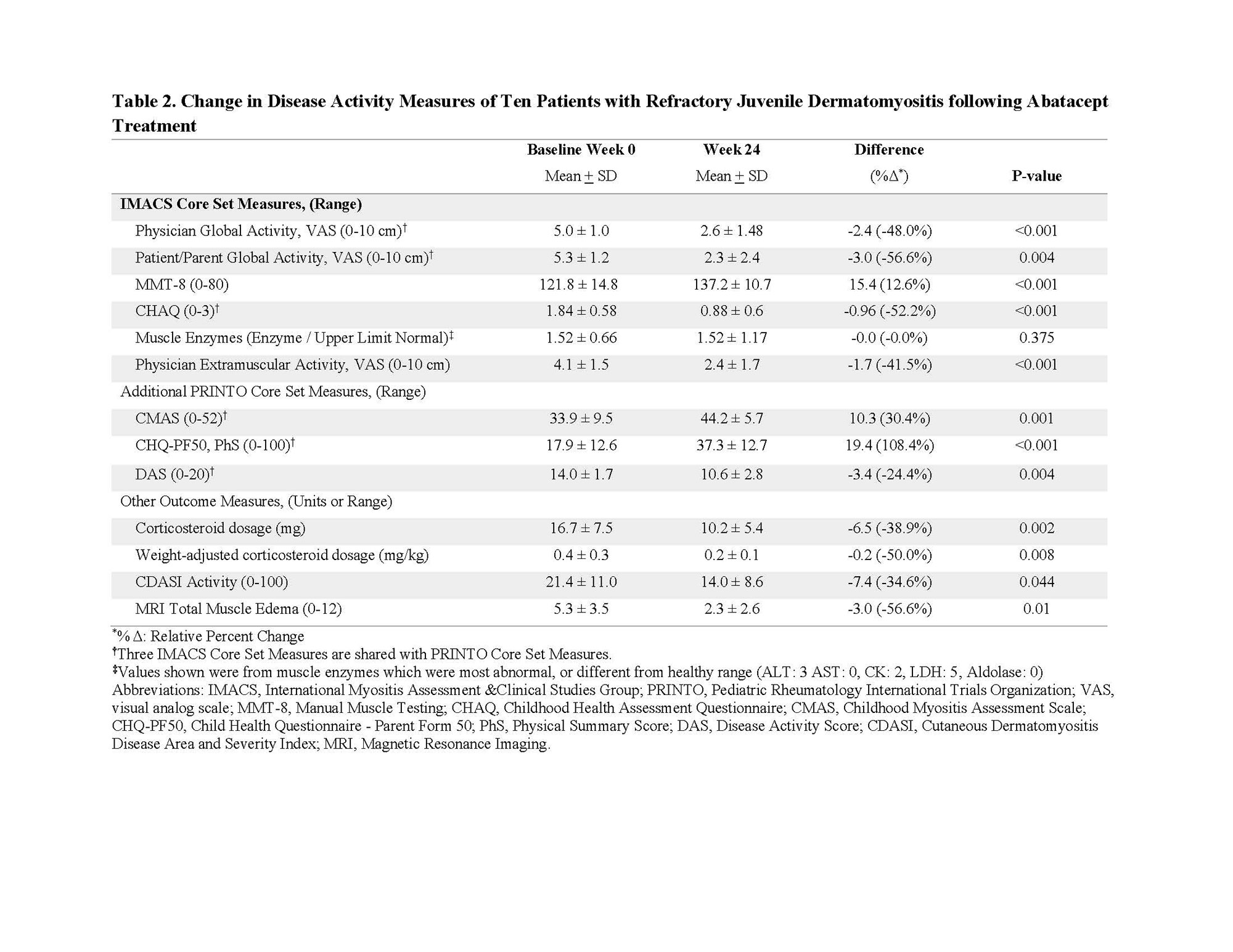
Results: At week 24, nine of 10 patients achieved DOI using IMACS core set measures (CSMs). Using PRINTO CSMs 4 patients met minimal and 5 met moderate improvement at week 24. The mean ACR-EULAR Myositis Response Criteria TIS using IMACS and PRINTO CSMs was 53.8±19.2 and 65.3±25.9, respectively at week 24. Three patients attained major, 4 attained moderate, and 2 minimal improvement at week 24 by the ACR-EULAR response criteria using IMACS CSMs. Seven of 10 patients had major, one had moderate, and one patient minimal improvement by ACR-EULAR response criteria using PRINTO CSMs (Table 1). All CSMs improved significantly (p< 0.05) from baseline to week 24 (13-57% relative percent change), except for muscle enzymes (Table 2). Daily corticosteroid dose significantly decreased from a mean of 16.7±7.5 mg at baseline to 10.2±5.4 mg at week 24, a decrease of 39%. There was a significant decrease in the skin Cutaneous Disease Area and Severity Index (CDASI) score from baseline (21.4±11.0) to week 24 (14.0±8.6) by -35%. Eight patients joined the open-label extension phase, and had a 41±4 weeks from baseline (Table 1). Seven of these patients maintained at least minimal improvement based on the IMACS DOI, while the remaining patient worsened, due to worsening in MMT-8. The MRI total muscle edema score was improved by 57% at week 24 (p=0.01), but muscle atrophy, fascial edema, and subcutaneous edema scores were unchanged. There were strong correlations (rs=0.81) between change in MRI muscle edema and change in IP-10/CXCL10 (p=0.009). Four grade 3 and 6 grade 2 treatment-emergent adverse events were observed. One patient discontinued therapy due to worsening ILD.
Conclusion: This investigational pilot study demonstrated abatacept may be beneficial for treatment refractory JDM. Controlled studies are needed.
Acknowledgements. We thank the following for research support: Bristol Myers Squib; Cure JM Foundation; GWU MFA; IRP of NIAMS including TIS and NIEHS, NIH and patients and their families for participation in the study.
To cite this abstract in AMA style:
Mamyrova G, Nguyen W, Awal H, Jones D, Ehrlich A, Brindle K, Haji-Momenian S, Sheets R, Chin A, Lu S, Gadina M, Kim H, Jones O, Rider L, Curiel R. Abatacept in the Treatment of Refractory Juvenile Dermatomyositis [abstract]. Arthritis Rheumatol. 2022; 74 (suppl 9). https://acrabstracts.org/abstract/abatacept-in-the-treatment-of-refractory-juvenile-dermatomyositis/. Accessed .« Back to ACR Convergence 2022
ACR Meeting Abstracts - https://acrabstracts.org/abstract/abatacept-in-the-treatment-of-refractory-juvenile-dermatomyositis/