Session Information
Date: Monday, November 11, 2019
Title: Pediatric Rheumatology – ePoster II: SLE, Juvenile Dermatomyositis, & Scleroderma
Session Type: Poster Session (Monday)
Session Time: 9:00AM-11:00AM
Background/Purpose: Juvenile dermatomyositis (JDM) is a rare, chronic autoimmune illness characterized by symmetric, proximal muscle damages and involvement of the skin. While first line treatment is based on a combination of DMARDs (Methotrexate or cyclosporin) and glucocorticoids, there is limited evidence on treatment strategies for patients unresponsive to first-line treatment. We assessed the efficacy/effectiveness and safety of biologic agents in JDM.
Methods:
A systematic literature review was conducted using Embase®, MEDLINE®, MEDLINE®-In Process and Cochrane library to identify studies on biologics agents in JDM published in English language as full-text articles (1975 to May 2019) or conference abstracts (2000 to May 2019).
Databases were searched with the key words “(juvenile dermatomyositis) crossed with “biologic agents OR tocilizumab OR rituximab OR adalimumab or INFLIXIMAB or anti TNF or baricitinib OR etanercept OR JAK inhibitors”. Of note, we did not include children, age, or age limits in the search as medical subject headings terms because we may have been able to extract a sub cohort of children from studies including both children and adults
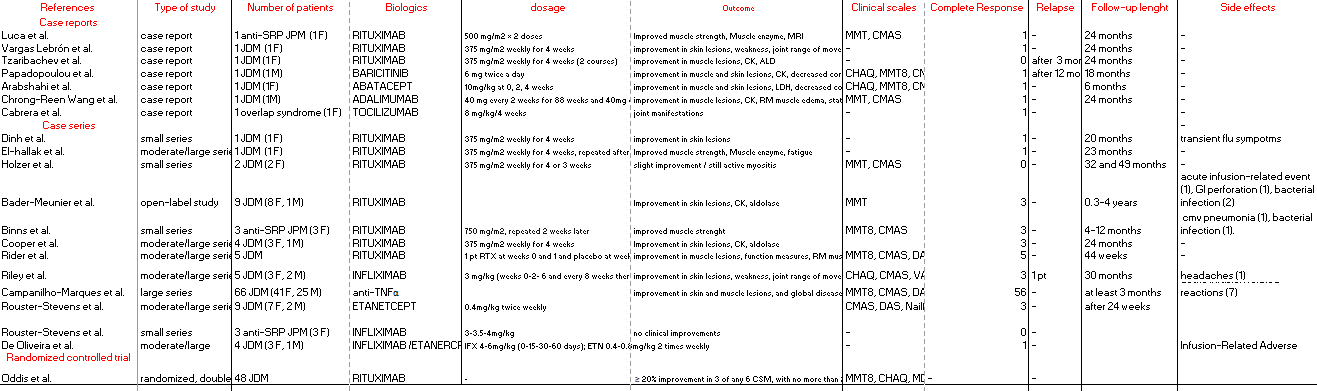
Results: Of the 1584 retrieved publications, 20 articles were identified for a total of 167 patients. 7 patients presented with anti-signal recognition particle polymyositis. Only one RCT was identified for Rituximab; however, in the Rituximab in Myositis (RIM) trial no pediatric data could be extracted since they were reported as aggregate. In real-world studies, complete (CR) or partial response (PR) was often assessed, and their definitions and follow-ups (FUP) varied (table 1). JDM pts were most often treated with anti-TNF (90 pts); 11 pts received etanercept (ETA), 12 pts Infliximab (IFX), 1 pt adalimumab (ADA). Data regarding specific anti-TNFa agents were not available for the other 66 pts, as were not extractable. Rituxumab (RTX) was used in 76 pts. Single case reports reported the use of tocilizumab (TCZ), abatacept (ABA) e baricitinib (Table 1). Complete response was reported for 72% of pts treated with at least one anti-TNF and 64% for RTX, without statistical difference in head-to-head drug comparison. Anti-TNF were generally well-tolerated but 9 pts experience severe reactions (8 infusion-related adverse reaction; 1 infection). In the RTX group 6 patients experience severe events (3 infections; 1 infusion-related adverse reaction; 1 gastrointestinal perforation).
Conclusion: Anti-TNF and RTX was efficacious in controlling disease in JDM in the studies assessed. However, response criteria and treatments were not directly comparable, thus more study are needed to determine the optimal treatment in the real-world setting
To cite this abstract in AMA style:
marrani e, Abu Rumeileh S, Tirelli F, Maccora I, Simonini G. A Systematic Literature Review of Efficacy and Safety of Biologic Agents for the Treatment of Juvenile Dermatomyositis [abstract]. Arthritis Rheumatol. 2019; 71 (suppl 10). https://acrabstracts.org/abstract/a-systematic-literature-review-of-efficacy-and-safety-of-biologic-agents-for-the-treatment-of-juvenile-dermatomyositis/. Accessed .« Back to 2019 ACR/ARP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/a-systematic-literature-review-of-efficacy-and-safety-of-biologic-agents-for-the-treatment-of-juvenile-dermatomyositis/