Session Information
Date: Monday, October 27, 2025
Title: (1553–1591) Systemic Sclerosis & Related Disorders – Clinical Poster II
Session Type: Poster Session B
Session Time: 10:30AM-12:30PM
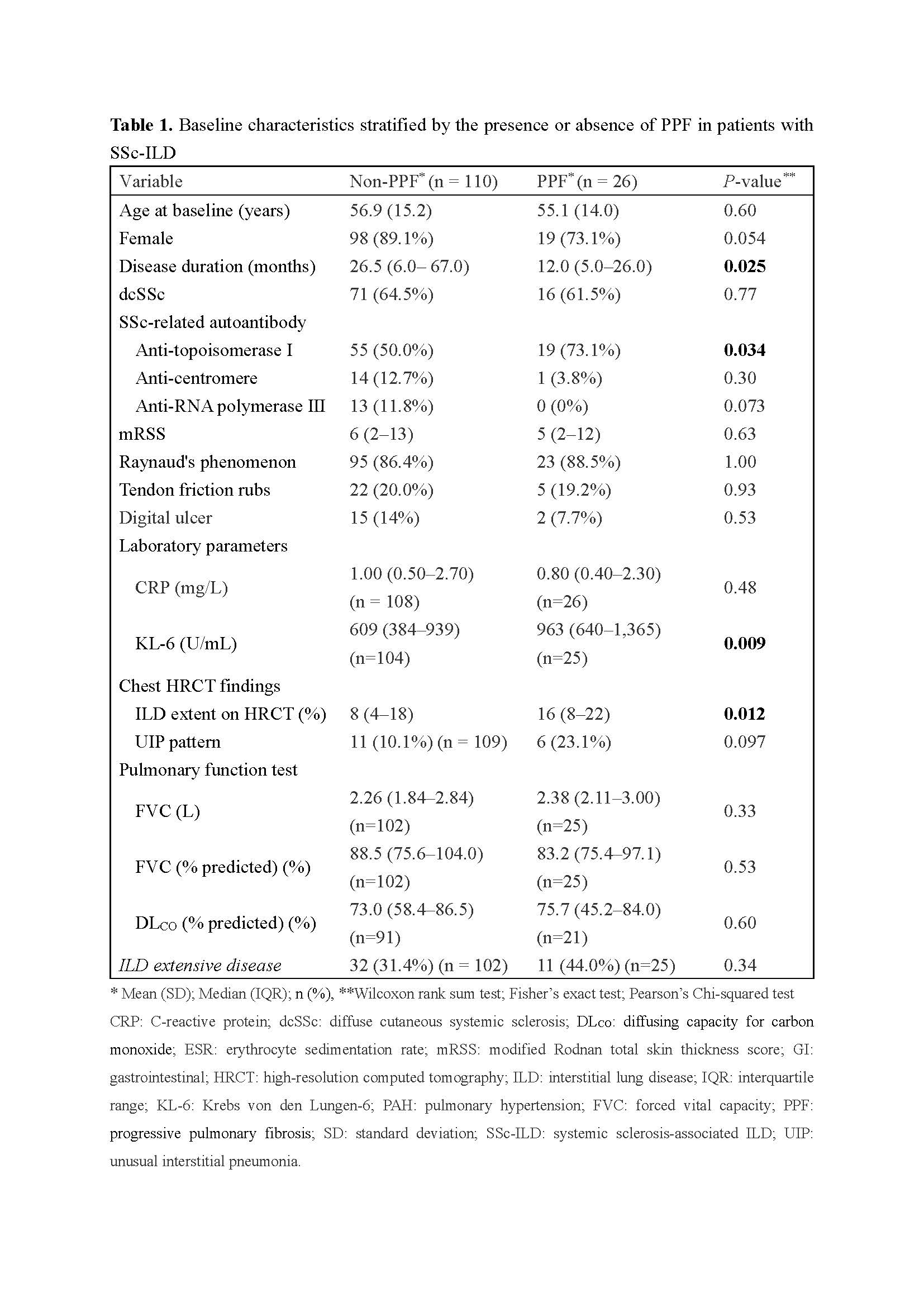
Background/Purpose: Interstitial lung disease (ILD) is leading cause of morbidity and mortality in patients with systemic sclerosis (SSc). Given the high variable clinical course of SSc-associated ILD (SSc-ILD), accurate prediction of ILD progression is crucial for both clinical practice and trial designing. Circulating biomarkers have emerged as convenient, non-invasive tools for the diagnosis and prognostication of SSc-ILD. Among these, elevated levels of Krebs von den Lungen-6 (KL-6), a marker for alveolar epithelial injury, has been consistently associated with subsequent decline in forced vital capacity (FVC) and progression to end-stage lung disease [1,2]. However, it remains unclear whether serial changes in KL-6 levels can predict ILD progression. This study aimed to determine whether rising KL-6 levels are predictive of progressive pulmonary fibrosis (PPF) in patients with SSc-ILD using a single-center prospective cohort.
Methods: A total of 136 patients with SSc-ILD were enrolled from a prospective SSc registry at Nippon Medical School Hospital. Patients were categorized into PPF and non-PPF groups based on the proposed criteria [3]. Baseline characteristics were compared between the groups, and a Cox proportional hazards model was used to identify baseline predictors of PPF. To assess the prognostic significance of dynamic KL-6 changes, a time-varying Cox model was applied using KL-6 measurements taken 12 and 6 months prior to either the first PPF event (PPF group) or the last follow-up visit (non-PPF group). KL-6 values were log2-transformed to facilitate interpretation per doubling. Models were adjusted for clinically relevant covariates.
Results: Over a median follow-up of 34 months, 26 patients (19%) developed PPF. Comparison to the non-PPF group, the PPF group had higher prevalence of anti-topoisomerase I antibody (P = 0.034), and a greater ILD extent on high-resolution computed tomography (HRCT) (P = 0.012). Baseline serum KL-6 levels were significantly higher in the PPF group (P = 0.009). In the Cox model, positive anti-topoisomerase I antibody was associated with 236% higher risk of future PPF development (HR: 3.36, 95% confidence interval (CI): 1.17-9.62, P = 0.024), while ILD extent, usual interstitial pneumonia (UIP) pattern on HRCT, and FVC didn’t show significant association. In the time-varying Cox model, a doubling of KL-6 levels was associated with 72% higher risk of subsequent PPF (HR 1.72; 95% CI 1.09–2.71; P = 0.020). This association remained significant and was even strengthened after adjusting for clinically relevant covariates, including anti-topoisomerase I antibody status (HR 2.17; 95% CI 1.06–4.45; P = 0.034).
Conclusion: A rise in serum KL-6 levels was significantly associated with the near-term development of PPF in patients with SSc-ILD. These findings support the use of serial KL-6 measurements as a non-invasive biomarker to monitor disease progression and guide clinical decision-making in SSc-ILD.Reference[1] J Rheumatol 2016; 43: 1825[2] Arthritis Rheumatol 2019; 71: 2059[3] Am J Respir Crit Care Med 2022; 205: e18
To cite this abstract in AMA style:
Yomono K, Huang S, Khanna D, Kuwana M. A rise in serum KL-6 levels predicts subsequent progressive pulmonary fibrosis in patients with systemic sclerosis-associated interstitial lung disease [abstract]. Arthritis Rheumatol. 2025; 77 (suppl 9). https://acrabstracts.org/abstract/a-rise-in-serum-kl-6-levels-predicts-subsequent-progressive-pulmonary-fibrosis-in-patients-with-systemic-sclerosis-associated-interstitial-lung-disease/. Accessed .« Back to ACR Convergence 2025
ACR Meeting Abstracts - https://acrabstracts.org/abstract/a-rise-in-serum-kl-6-levels-predicts-subsequent-progressive-pulmonary-fibrosis-in-patients-with-systemic-sclerosis-associated-interstitial-lung-disease/