Session Information
Session Type: Poster Session B
Session Time: 10:30AM-12:30PM
Background/Purpose: Glucagon-like peptide-1 receptor agonists (GLP1-RA) are an emerging class of medications that significantly improve cardiometabolic outcomes. Whether these drugs may be useful in mitigating the metabolic dysfunction associated with corticosteroid use and the pathogenesis of SLE remains unknown, and the safety of GLP1-RAs in SLE patients has yet to be established. A recent case report describing drug induced lupus, characterized by anti-dsDNA and anti-histone antibodies, secondary to GLP1-RA administration underscores these uncertainties and may cause some providers to limit the use of these drugs in SLE patients (1). Accordingly, a retrospective analysis of the multiracial/multiethnic NYU Lupus cohort was performed to evaluate outcomes of GLP1-RAs in SLE.
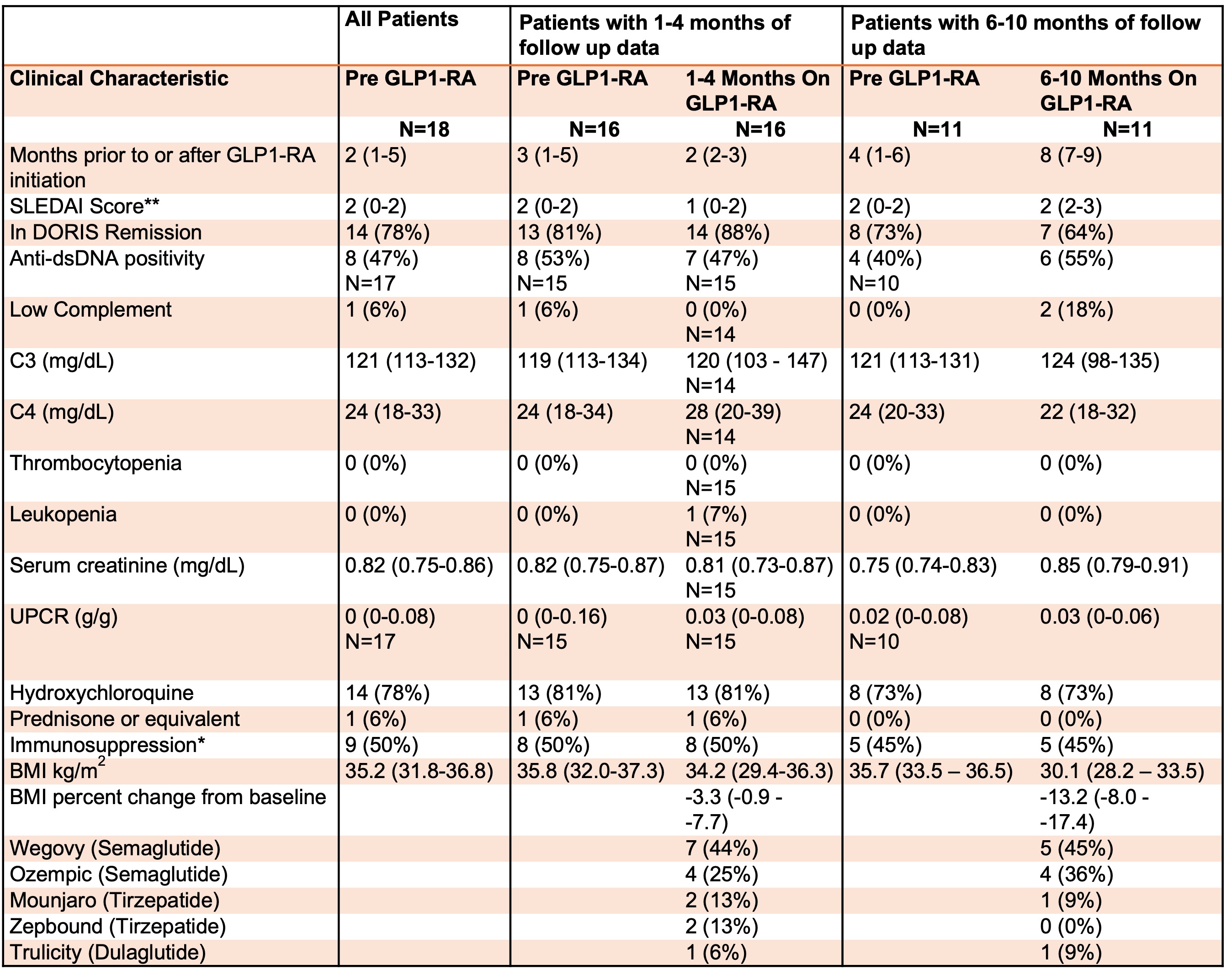
Methods: Since 2014 patients fulfilling either ACR, SLICC, and/or EULAR criteria for SLE, speaking English, Spanish, and Mandarin, are consented into the NYU Lupus cohort at a routine rheumatology visit. Comprehensive clinical data including medications and disease activity are recorded at each visit thereafter. This database was queried for all GLP1-RAs to identify relevant cases. Clinical characteristics of patients were assessed at baseline (most recent rheumatology visit prior to starting GLP1-RA), 1-4 months, and 6-10 months after GLP1-RA initiation based on available follow up data.
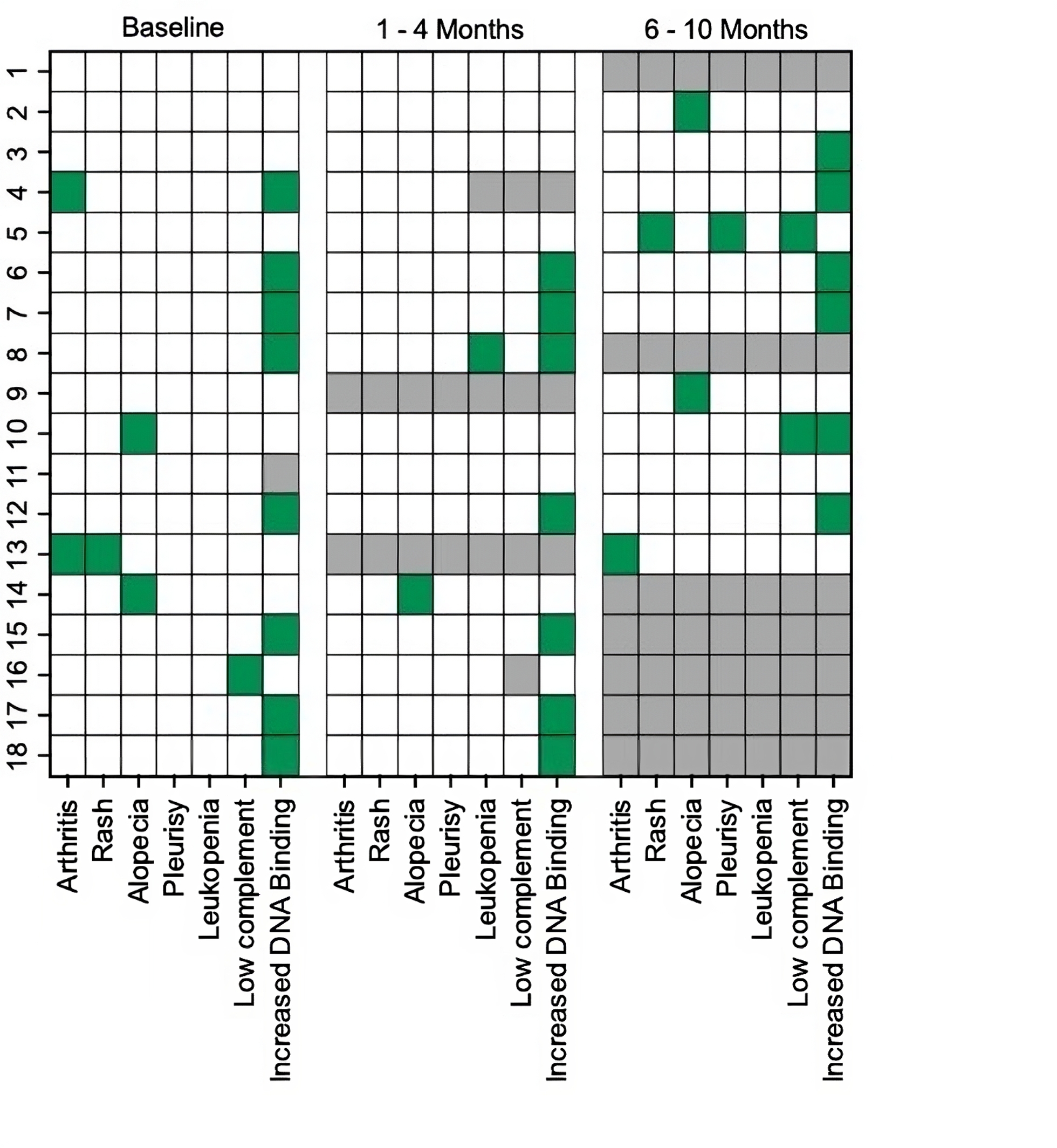
Results: Of the 1211 patients in the cohort, only 24 had received a GLP1-RA. Six were excluded from the analysis due to insufficient documentation regarding the duration of medication use. Of the remaining 18 (median age 50), 17 (94%) were female, 9 (50%) were White, 7 (39%) were Black, and 2 (11%) were Hispanic. All patients were on GLP1-RAs for weight loss and only one had type 2 diabetes. In no case was the prescription written by rheumatology (6 (33%) weight management, 7 (39%) endocrinology, 2 (11%) bariatrics, 2 (11%) primary care, 1 (6%) unknown). At baseline, all but four patients were in DORIS remission and no patients had active nephritis. There was follow up data available for 16 patients at 1-4 months and 11 patients at 6-10 months. There was one mild to moderate flare assessed by the SELENA-SLEDAI flare index at 8 months characterized by recurrent pleurisy, rash, and low complement; otherwise, no other flares were observed at follow up (Table 1 and Figure 1). In total, three (27%) patients developed recurrent low complement and/or anti-dsDNA antibodies at 6-10 months. There was a median reduction in BMI of 13% from baseline at 6-10 months (p=0.001) (Table 1). Nine (50%) patients were initially denied insurance coverage for a GLP1-RA, but all received coverage after appealing or switching to a different agent in the same class.
Conclusion: While limited by a small sample size, this descriptive study showed that in SLE patients with overall low baseline disease activity, GLP1-RAs did not trigger new clinical SLE manifestations and associated with improved metabolic dysfunction through decreased BMI. Despite their potential usefulness as adjunctive agents for lowering cardiometabolic risk, several patients faced initial insurance rejections for GLP1-RAs, which may pose a major barrier to their routine implementation by rheumatologists.
To cite this abstract in AMA style:
Carlucci P, Cohen B, Saxena A, Belmont H, Masson M, Gold H, Buyon J, Izmirly P. A Retrospective Evaluation of Glucagon-like Peptide-1 Receptor Agonists in SLE Patients [abstract]. Arthritis Rheumatol. 2024; 76 (suppl 9). https://acrabstracts.org/abstract/a-retrospective-evaluation-of-glucagon-like-peptide-1-receptor-agonists-in-sle-patients/. Accessed .« Back to ACR Convergence 2024
ACR Meeting Abstracts - https://acrabstracts.org/abstract/a-retrospective-evaluation-of-glucagon-like-peptide-1-receptor-agonists-in-sle-patients/