Session Information
Date: Sunday, November 12, 2023
Title: Abstracts: RA – Treatments I: Novel RA Treatments & Mechanisms of Action
Session Type: Abstract Session
Session Time: 4:00PM-5:30PM
Background/Purpose: Vagus nerve stimulation (VNS) has emerged in recent decades as a potential therapy for RA.We have previously shown that auricular VNS produced an average reduction of 1.4 in the disease activity score of 28 joints with C-reactive protein (DAS28-CRP) and significant improvements in the American College of Rheumatology (ACR) responses in an open-label study of 30 patients with active RA. Similarly, studies with an implantable cervical vagus nerve stimulator have shown reduced signs and symptoms of RA. However, studies to date have been relatively small and/or uncontrolled. The purpose of this study was to investigate the safety and efficacy of auricular vagus nerve stimulation for the treatment of RA in a randomized, double-blind, sham-controlled study.
Methods: This randomized, double-blind, sham-controlled trial enrolled patients aged 18-75 years with active RA who had failed or were intolerant of conventional synthetic disease-modifying antirheumatic drugs (csDMARDs) and were naïve to biologic and/or targeted synthetic DMARDs. All patients received an auricular vagus nerve stimulator and were randomized 1:1 to active stimulation or sham. The primary endpoint was the proportion of patients achieving 20% improvement in American College of Rheumatology criteria (ACR20) at week 12. Secondary endpoints included mean changes in disease activity score of 28 joints with C-reactive protein (DAS28-CRP) and Health Assessment Questionnaire-Disability Index (HAQ-DI).
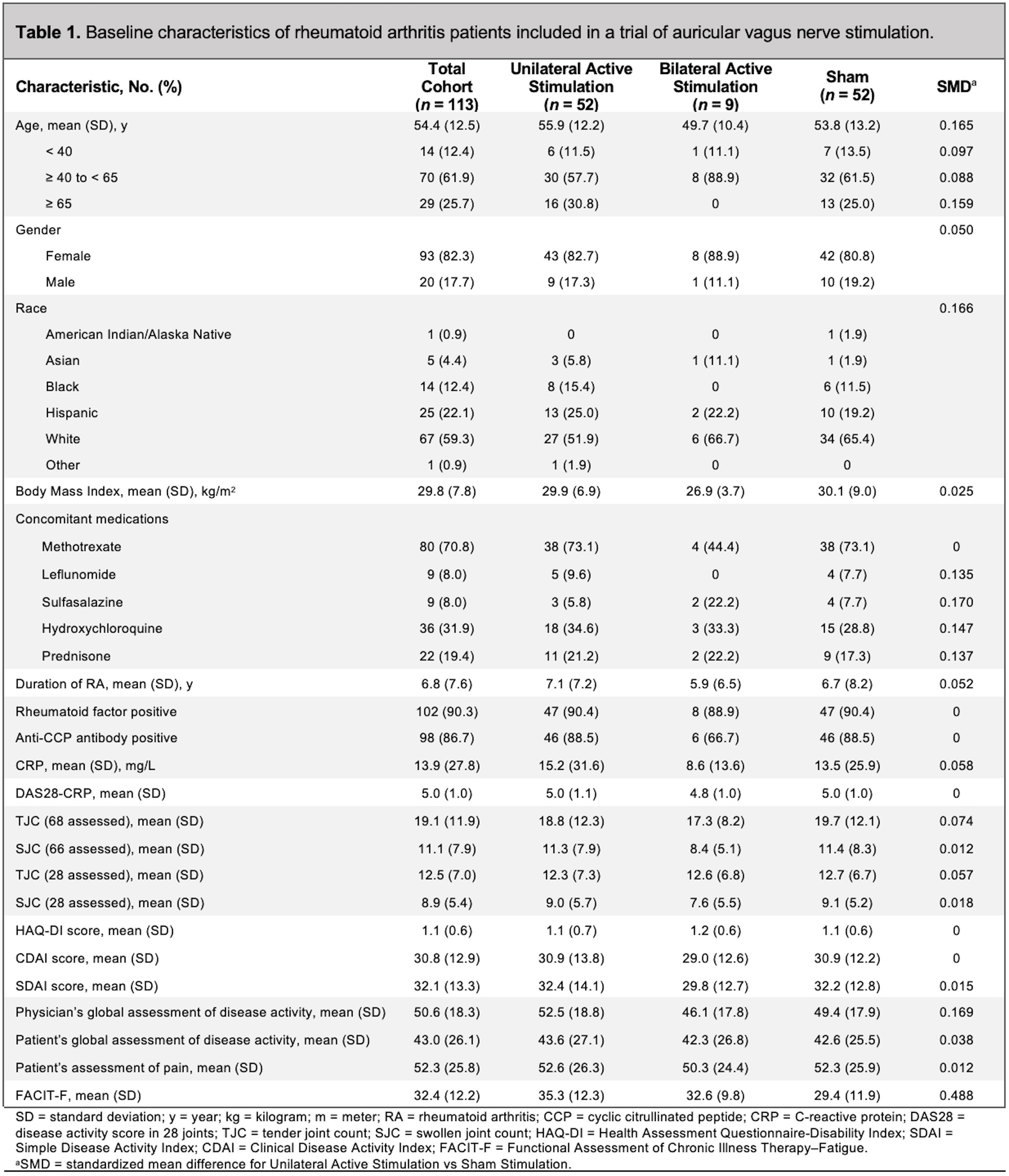
Results: A total of 113 patients received study therapy, and 101 patients (89.4%) completed the study visits through week 12. Baseline characteristics are shown in Table 1.
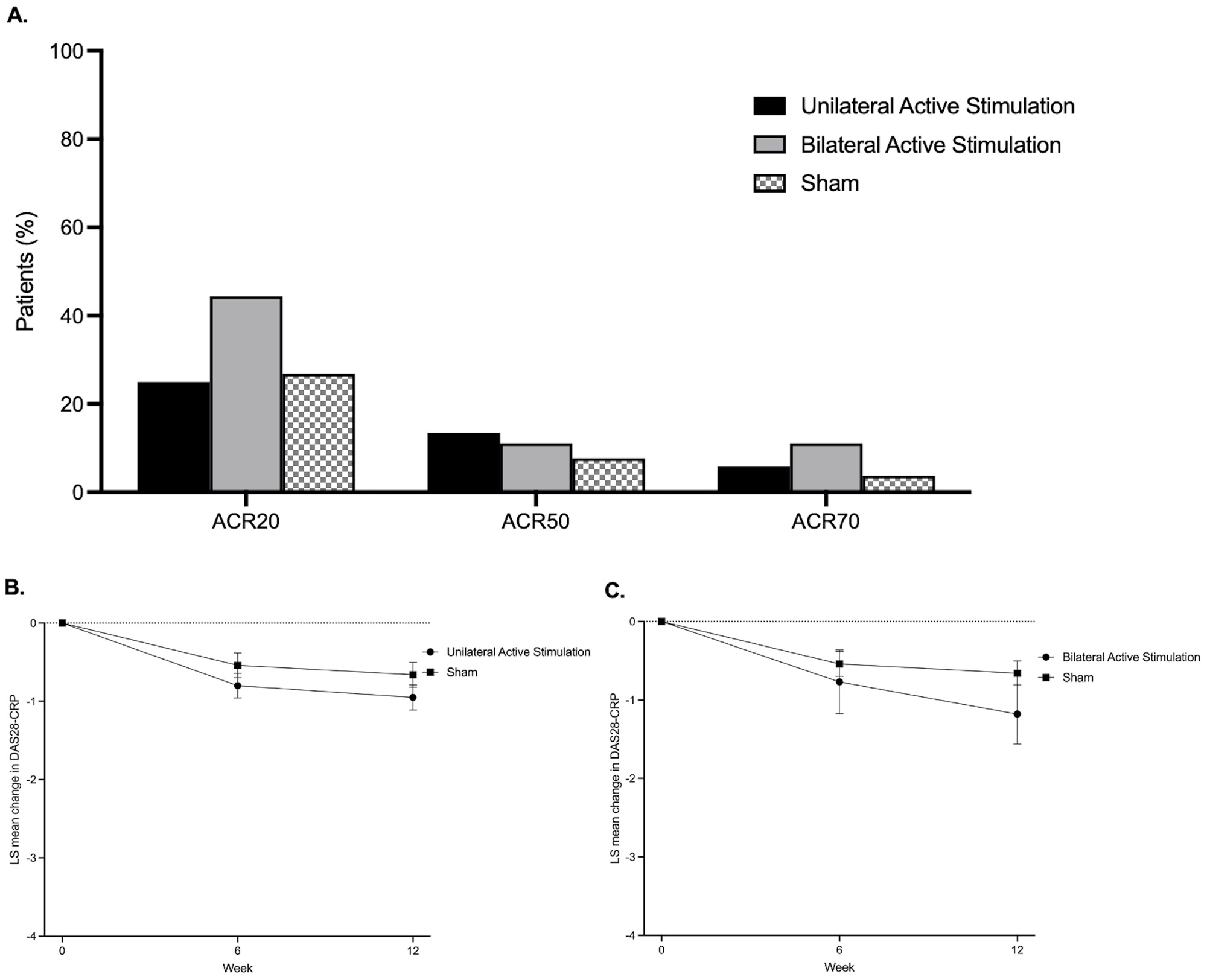
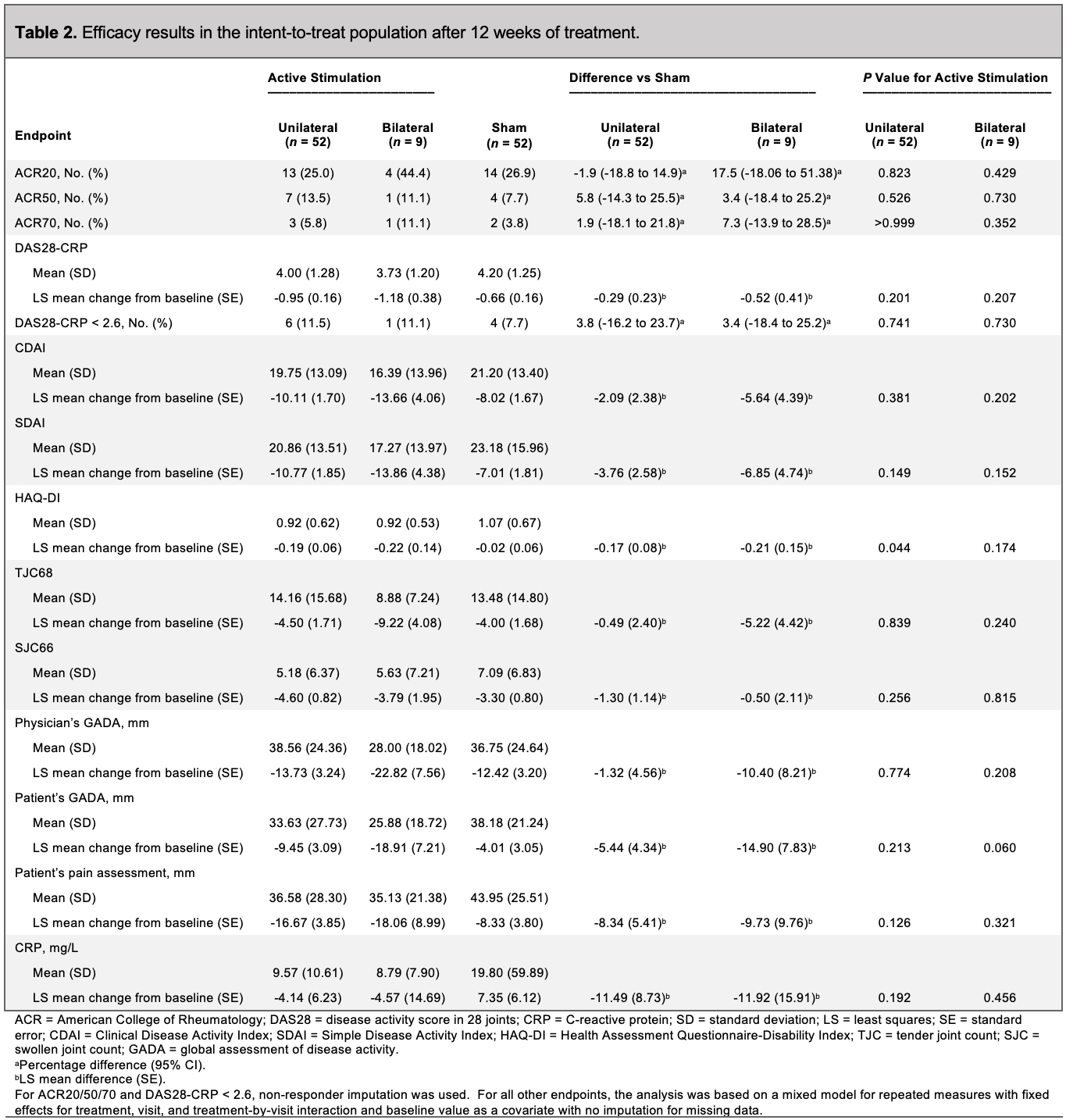
ACR20 responses at week 12 were not significantly different in patients receiving active unilateral stimulation versus sham: 25.0% for active stimulation vs 26.9% for sham (difference vs sham: -1.9% [-18.8% to 14.9%]; p=0.823) (Table 2 and Figure). The LS mean (SE) changes in DAS28-CRP from baseline to week 12 were –0.95 (0.16) for active unilateral stimulation and –0.66 (0.16) for sham, with a difference in LS means (SE) vs sham of –0.29 (0.23) (p=0.201) (Figure). The LS mean (SE) changes in HAQ-DI from baseline to week 12 were –0.19 (0.06) for active unilateral stimulation and –0.02 (0.06) for sham, with a difference in LS means (SE) vs sham of –0.17 (0.08) (p=0.044). No significant differences were seen in DAS28-CRP of 2.6 or less, ACR50, ACR70, CDAI, or SDAI between the unilateral active stimulation and sham groups (Table 2, Figure).
Treatment-emergent AEs were reported in 10 patients (18.9%) receiving unilateral active stimulation, 2 patients (22.2%) receiving bilateral active stimulation, and 5 patients (9.8%) receiving sham. No AEs were grade 3 or greater in severity by Common Terminology Criteria for Adverse Events, and no serious adverse events (SAEs) or deaths occurred.
Conclusion: Auricular VNS was safe and well tolerated, but it did not meaningfully improve RA disease activity. More large, controlled studies of VNS for the treatment of RA are needed to better understand its potential future role.
To cite this abstract in AMA style:
Baker M, Kavanagh S, Cohen S, Matsumoto A, Dikranian A, Tesser J, Kivitz A, Alataris K, Genovese M. A Randomized, Double-Blind, Sham-Controlled, Clinical Trial of Auricular Vagus Nerve Stimulation for the Treatment of Active Rheumatoid Arthritis [abstract]. Arthritis Rheumatol. 2023; 75 (suppl 9). https://acrabstracts.org/abstract/a-randomized-double-blind-sham-controlled-clinical-trial-of-auricular-vagus-nerve-stimulation-for-the-treatment-of-active-rheumatoid-arthritis/. Accessed .« Back to ACR Convergence 2023
ACR Meeting Abstracts - https://acrabstracts.org/abstract/a-randomized-double-blind-sham-controlled-clinical-trial-of-auricular-vagus-nerve-stimulation-for-the-treatment-of-active-rheumatoid-arthritis/