Session Information
Date: Saturday, November 7, 2020
Title: RA – Treatments Poster II: Comparative Effectiveness, Biosimilars, Adherence & the Real World
Session Type: Poster Session B
Session Time: 9:00AM-11:00AM
Background/Purpose: CT-P17 (100 mg/mL) is the first proposed biosimilar of the high concentration and citrate-free formulation of reference adalimumab. The purpose of this study was to compare the efficacy and safety of CT-P17 with reference adalimumab in patients with active moderate-to-severe RA up to Week 52.
Methods: Patients with active moderate-to-severe RA despite methotrexate treatment were randomly assigned to receive 40 mg of CT-P17 or reference adalimumab every 2 weeks up to Week 24. Prior to dosing at Week 26, patients in the reference adalimumab group were re-randomized either to continue receiving reference adalimumab or to switch to CT-P17 until end of study. All patients initially randomized to CT-P17 continued CT-P17. The primary endpoint was ACR20 response rate at Week 24. Secondary measures of efficacy, pharmacokinetics (PK) and safety, including immunogenicity, were also evaluated.
Results: 648 patients initiated treatment (324 patients in each arm). Baseline characteristics were similar between groups.
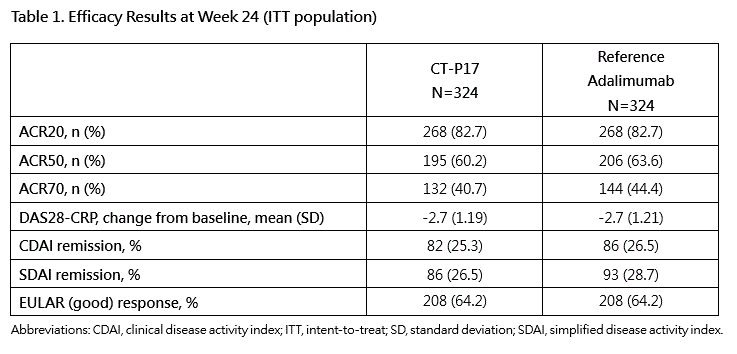
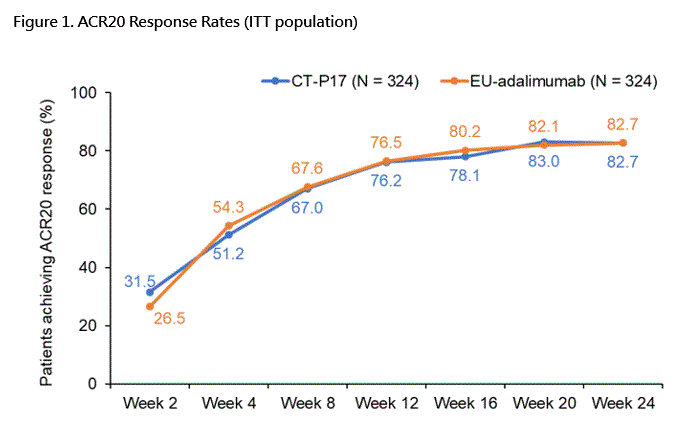
The ACR20 response rate at Week 24 was 82.7% (268/324) and 82.7% (268/324) for CT-P17 and reference adalimumab and the confidence intervals of the treatment difference (95% CI: -5.94 to 5.94, 90% CI: -4.98 to 4.98) were entirely within the equivalence margins agreed upon with EMA (±15%) and FDA (-12% to +15%). Secondary efficacy endpoints were also similar between groups (Table 1).
In terms of PK, geometric mean trough serum concentration was slightly higher in CT-P17; 5.3 μg/mL in CT-P17 vs. 4.3 μg/mL in reference adalimumab at Week 24 (p=0.0501).
Overall, 353 (CT-P17: 169 [52.2%] vs. reference adalimumab: 184 [56.8%]) patients experienced at least 1 treatment-emergent adverse event (TEAE). The most common TEAEs were injection site reactions (16 [4.9%] vs. 22 [6.8%]). 26 patients experienced at least 1 treatment-emergent serious adverse event (TESAE). Similar proportions of patients in both groups experienced at least 1 TEAE that was classified as hypersensitivity/allergic reactions and infections (Table 2). One malignancy (breast cancer; unrelated) was reported in a patient receiving CT-P17.
The proportion of patients who had anti-drug antibodies (ADAs) at Week 24 (209 [32.3%]), was slightly lower for CT-P17 (93 [28.7%]) than for reference adalimumab (116 [35.8%]) (p=0.0643). Of these, 83 (25.6%) receiving CT-P17 and 103 (31.8%) receiving reference adalimumab also had neutralizing ADAs.
Conclusion: Over 24 weeks, CT-P17 has equivalent efficacy to reference adalimumab, with ACR20 response rates of 82.7% for each, and similar additional secondary efficacy endpoints. Considering the ACR20 response rate of 64.0% – 82.5% [1-4] reported in previous studies of low concentration (50 mg/mL) adalimumab biosimilar, further investigation is needed to ascertain the factors associated with the slightly higher response rate in this study.
CT-P17 was well tolerated with a safety profile comparable to that of reference adalimumab.
References:
- Cohen SB et al. Ann Rheum Dis. 2017 Oct; 76(10):1679-1687
- Cohen SB et al. Ann Rheum Dis.2018 Jun; 77(6):914-921
- Weinblatt ME, Arthritis Rheumatol. 2018 Jan; 70(1):40-48
- US FDA, Multi-disciplinary Evaluation and Review for PF-06410293, 2019
To cite this abstract in AMA style:
Kay J, Jaworski J, Wojciechowski R, Wiland P, Dudek A, Krogulec M, Jeka S, Zielinska A, Trefler J, Bartnicka-Maslowska K, Krajewska-Wlodarczyk M, Klimiuk P, Furst* D, Lee S, Bae Y, Yang G, Yoo J, Lee H, Keystone E. A Randomized, Double-Blind, Phase 3 Study to Compare the Efficacy and Safety of a Proposed High Concentration (100 mg/mL) Adalimumab Biosimilar (CT-P17) with Reference Adalimumab in Patients with Moderate-to-Severe Active Rheumatoid Arthritis [abstract]. Arthritis Rheumatol. 2020; 72 (suppl 10). https://acrabstracts.org/abstract/a-randomized-double-blind-phase-3-study-to-compare-the-efficacy-and-safety-of-a-proposed-high-concentration-100-mg-ml-adalimumab-biosimilar-ct-p17-with-reference-adalimumab-in-patients-with-mode/. Accessed .« Back to ACR Convergence 2020
ACR Meeting Abstracts - https://acrabstracts.org/abstract/a-randomized-double-blind-phase-3-study-to-compare-the-efficacy-and-safety-of-a-proposed-high-concentration-100-mg-ml-adalimumab-biosimilar-ct-p17-with-reference-adalimumab-in-patients-with-mode/