Session Information
Date: Wednesday, November 15, 2023
Title: Abstracts: Systemic Sclerosis & Related Disorders III: Clinical Trials
Session Type: Abstract Session
Session Time: 11:00AM-12:30PM
Background/Purpose: Interstitial lung disease in systemic sclerosis(SSc-ILD) is heterogeneous with limited therapeutic options. Mycophenolate mofetil (MMF) is the most commonly used first line agent for SSc-ILD. Tacrolimus has shown promising efficacy in few small case series, and large cohorts of patients with non-SSC-ILD1,but has never been evaluated in the setting of a clinical trial in scleroderma.
Methods: In this single center open labelled, prospective, two-arm parallel group, randomized controlled pilot study (INSIST) conducted between November 2021 to December 2022, patients with progressive ILD (FVC decline >10%) due to SSc, aged between 18-65 years, disease duration < 10 years, without concomitant inflammatory myositis, with an FVC of 40-85%, and not having received active immunosuppression other than prednisolone < 10mg/day in the last 6 months were randomized to receive either MMF (target dose 2gm/day) or tacrolimus ( Max dose-0.075 mg/kg/day; target trough levels- 4- 10ng/ml) for 24 weeks. The primary endpoint was the difference in change in FVC% at 24 weeks; secondary outcomes included absolute change in FVC, skin scores, 6-minute walk distance, Mahler’s transitional dyspnea index, ACR-CRISS and revised CRISS responses and adverse outcomes. (Trial Reg: CTRI/2021/11/037864)
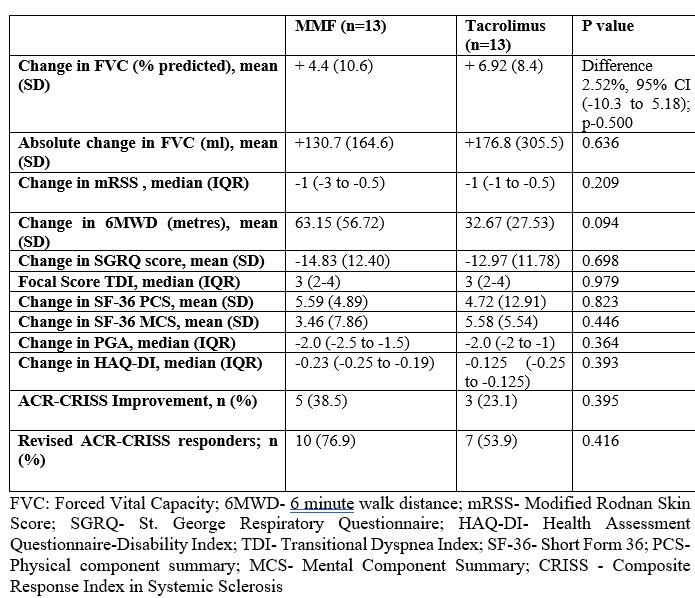
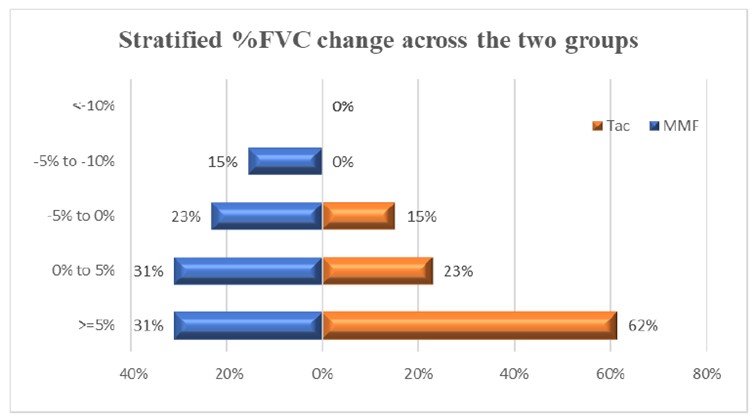
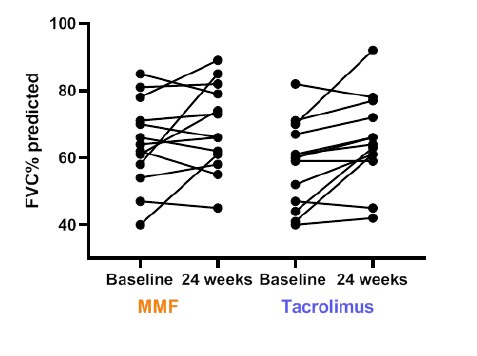
Results: 25 out of 26 patients (13 in each group) completed 24 weeks follow up. Majority had Anti-Scl 70 positivity (73%) and limited skin disease. At 24 weeks, the mean change in FVC was 4.4% (10.6) and 6.92%(8.4) in the MMF and tacrolimus groups respectively (difference 2.52%, 95% CI (-10.3 to 5.18); p-0.500). All patients on tacrolimus and 85% of patients on MMF had stabilization (ΔFVC% -5% TO 5%) or improvement (ΔFVC% >10%) in lung function. Secondary outcomes were similar between two groups. Subgroup analyses stratified by ILD type, skin involvement at baseline, and early vs late SSc yielded similar results. The mean tacrolimus levels were 4.9 ng/ml(1.47) and the median dose needed to achieve these levels was 4mg/d. No serious adverse events were noted in either group. Gastrointestinal disturbances was noted in 23% of patients of mycophenolate and new onset hypertension, minor infection and diarrhea in 1 patient each in the tacrolimus arm. Notably, tacrolimus did not result in renal dysfunction or renal crises.
Conclusion: Tacrolimus resulted in comparable improvement to MMF across primary and secondary outcome measures at 24 weeks with a favorable safety profile in patients with SSc ILD. Larger studies with longer follow up are needed to investigate the role of calcineurin inhibitors in SSc.
To cite this abstract in AMA style:
Sharma S, Mathew J, Kopp C, Dhir V, Dhooria S, Sinha A, Jain S. A Randomized Controlled Trial to Compare the Efficacy and Safety of Tacrolimus with Mycophenolate Mofetil in Patients with Systemic Sclerosis – Interstitial Lung Disease ( INSIST TRIAL) [abstract]. Arthritis Rheumatol. 2023; 75 (suppl 9). https://acrabstracts.org/abstract/a-randomized-controlled-trial-to-compare-the-efficacy-and-safety-of-tacrolimus-with-mycophenolate-mofetil-in-patients-with-systemic-sclerosis-interstitial-lung-disease-insist-trial/. Accessed .« Back to ACR Convergence 2023
ACR Meeting Abstracts - https://acrabstracts.org/abstract/a-randomized-controlled-trial-to-compare-the-efficacy-and-safety-of-tacrolimus-with-mycophenolate-mofetil-in-patients-with-systemic-sclerosis-interstitial-lung-disease-insist-trial/