Session Information
Date: Sunday, October 26, 2025
Title: (0671–0710) Systemic Sclerosis & Related Disorders – Clinical Poster I
Session Type: Poster Session A
Session Time: 10:30AM-12:30PM
Background/Purpose: Mycophenolate mofetil (MMF) is recommended for skin in diffuse cutaneous (dc)SSc, and for lung fibrosis in SSc, but patients with limited cutaneous (lc)SSc are not routinely started on MMF at diagnosis. This may miss an opportunity to prevent or treat major complications early. MINIMISE-Pilot tested feasibility of a randomised event-driven trial of MMF in lcSSc.
Methods: We explored recruitment and feasibility of a trial evaluating impact of MMF on a novel event-driven composite endpoint. The MINIMISE endpoint measures time to clinical worsening of lcSSc determined by one of the following: progressive lung fibrosis, pulmonary hypertension, scleroderma renal crisis, heart failure, severe gut involvement, major digital vascular complications, or death. Subjects were stratified by anticentromere antibody (ACA) status.This study prioritised remote assessment and used the novel Patient self-Assessment of Skin Thickness of Upper Limb (PASTUL) questionnaire to measure skin fibrosis. Primary outcomes were safety and feasibility. Pre-specified “go and no-go” criteria were based on patient number and event rate with agreed thresholds of success.
Results: Recruitment was challenging and a total of 53 subjects were screened and 43 randomised, 21 to the MMF arm (Figure 1). Average age of participants was 57 years, with near equal distribution between the control and MMF groups. Baseline characteristics and treatment allocation are summarised in Table 1. Most participants were female (84%), of White British ethnicity (81%) and had positive ACA (67%). Since recruitment was below 60 participants, MINIMISE-Pilot was terminated based upon the prespecified threshold for continuation.There were no major safety concerns. Serious Adverse Events (SAEs) occurred in 3 patients (7%). These included 2 (9%) in the control group and one (5%) in the MMF group. SAEs reported were gastrointestinal disorders (1 on MMF), injury and procedural complications (1 control), and musculoskeletal and connective tissue disorders (1 control). During the treatment period no participants experienced clinical worsening endpoints. Adherence to MMF was generally high, with 19 participants (95%) being 100% adherent at Week 1, decreasing to 9 participants (64%) at Week 24.
Conclusion: This study achieved its key goal as a feasibility pilot leading to early termination of the study due to low recruitment. This precludes interpretation of effectiveness and safety of MMF in lcSSc. Whilst emerging evidence strongly supports the use of MMF to treat skin and lung fibrosis in SSc, and other studies have validated the novel composite MINIMISE endpoint of “clinical worsening in lcSSc” and PASTUL endpoint for skin, the results of MINIMSE-Pilot suggest that a randomised prospective trial across 8 active sites in UK with relatively short duration is not feasible. This is important and will inform design of future studies testing benefit of MMF in lcSSc. Our previous power calculations suggest that around 400 patients and a treatment period of 36 months would be needed for the current study design, although emerging enrichment strategies may reduce the required subject number.
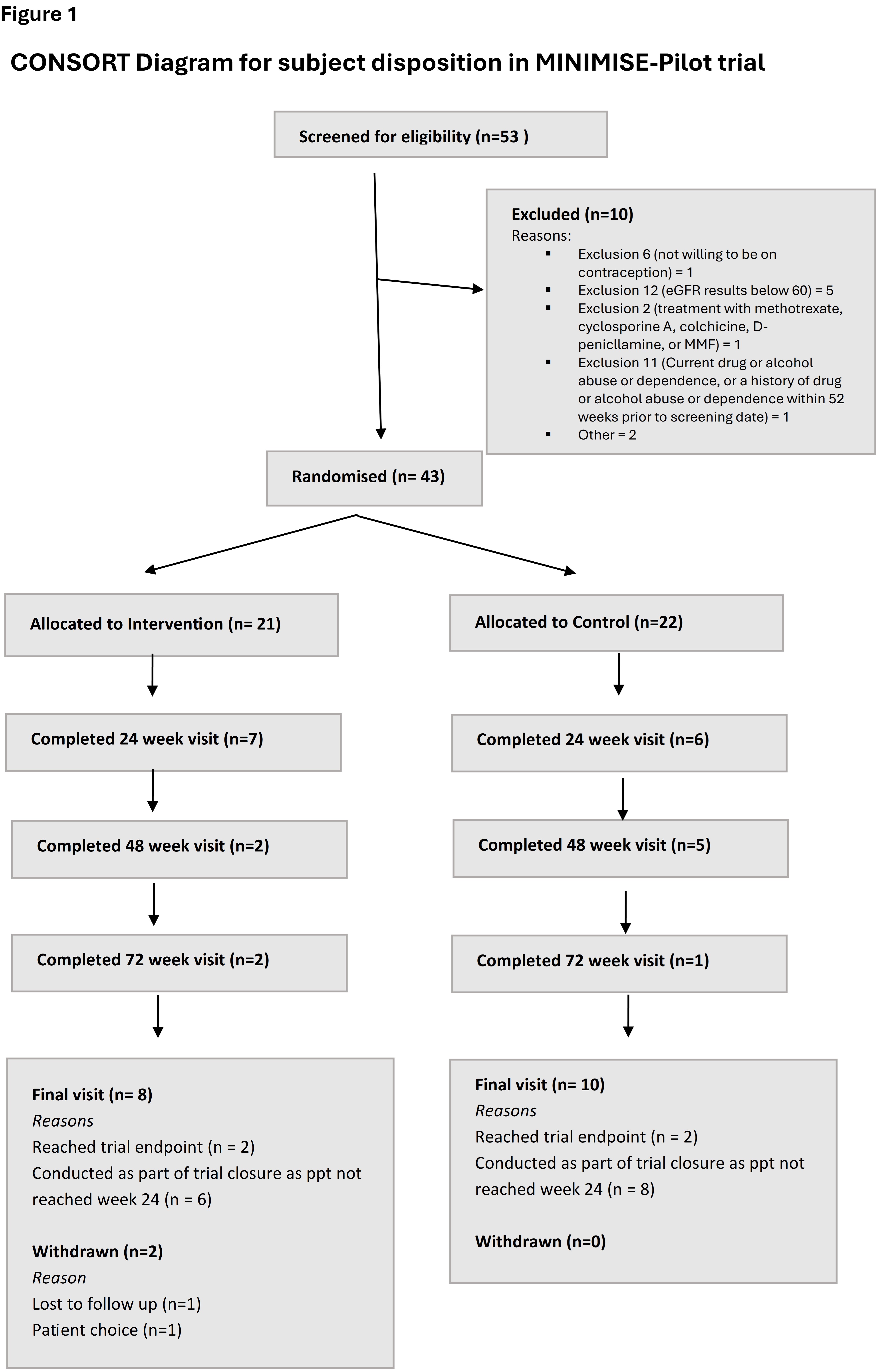 Figure 1 Subject disposition in MINIMISE-Pilot trial
Figure 1 Subject disposition in MINIMISE-Pilot trial
.jpg) Table 1 Baseline characteristics by treatment allocation
Table 1 Baseline characteristics by treatment allocation
To cite this abstract in AMA style:
Denton C, Yee P, kanitkar m, Clarke C, Ahmed S, Ong V, Del Galdo F, Pauling J, Anderson M, Samaranayaka M, Hughes M, Bhat S, Griffiths B, BUCH M, D'Cruz D, Herrick A, Vonk M, Feemantle N, Hakim-Moulay D. A Randomised Open Label Pilot Trial Comparing Mycophenolate Mofetil with no Immunosuppression in Limited Cutaneous Systemic Sclerosis (MINIMISE-Pilot) [abstract]. Arthritis Rheumatol. 2025; 77 (suppl 9). https://acrabstracts.org/abstract/a-randomised-open-label-pilot-trial-comparing-mycophenolate-mofetil-with-no-immunosuppression-in-limited-cutaneous-systemic-sclerosis-minimise-pilot/. Accessed .« Back to ACR Convergence 2025
ACR Meeting Abstracts - https://acrabstracts.org/abstract/a-randomised-open-label-pilot-trial-comparing-mycophenolate-mofetil-with-no-immunosuppression-in-limited-cutaneous-systemic-sclerosis-minimise-pilot/
