Session Information
Session Type: Poster Session B
Session Time: 10:30AM-12:30PM
Background/Purpose: Many patients with systemic lupus erythematosus (SLE) require teratogenic medications for management of their disease. Reliable contraception is important to avoid unintended pregnancy in this population, but contraception is inconsistently discussed and documented in the electronic medical record (EMR). The goal of this quality improvement intervention was to improve contraception discussions via a streamlined outreach campaign.
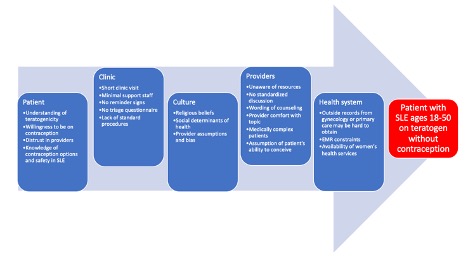
Methods: In Fall 2023, patients in an academic rheumatology clinic age 18-50 with SLE with a prescription for mycophenolate mofetil, mycophenolic acid, methotrexate, leflunomide, or cyclophosphamide were identified using an EMR tool. A detailed chart review was conducted to identify documentation of contraception. A process map and root cause analysis were completed to understand reasons for inadequate discussion of contraception. Provider comfort and lack of standard procedures were the main barriers identified (Figure 1). In February 2024, an EMR message was sent to patients without clear contraception documentation, to those documented to not be on contraception, and to those documented to have contraception considered potentially less reliable, such as condoms or abstinence. Patients who did not respond to the EMR message received a phone call. Responses were documented in the EMR and forwarded to the patient’s primary rheumatologist. The primary outcome measure was a 10% increase in documentation of contraception by April 2024. A secondary goal was to clarify contraception methods for those documented to be using less reliable contraception.
Results: We identified 119 patients with SLE ages 18-50 prescribed a teratogenic medication after exclusions. Of those, 43 (36%) had documentation of contraception in a rheumatology note within the past 18 months, 52 (44%) had documentation elsewhere in the EMR but not in a rheumatology note, and 24 (20%) had no clear EMR documentation. A total of 45 (38%) patients without clear EMR documentation (N = 24) or use of less reliable contraception or no contraception (N = 21) received outreach. Of the 24 patients without clear documentation, 13 (11% of the original 119) had updated documentation of contraception. For the 21 patients with less reliable forms of contraception, after intervention, contraception type was updated for 8 patients (7%). Through the process, 2 patients were referred to gynecology for long acting reversible contraception and 2 patients initiated discussions with their provider about SLE medication management while pursuing pregnancy.
Conclusion: Conception documentation is a measurable proxy for ensuring discussion of contraception in patients with SLE on teratogenic medications. Streamlined outreach to patients with SLE on teratogenic medication led to improved contraception documentation and led to referrals for contraception initiation and medication discussions for patients pursuing pregnancy. Easily accessible EMR tools can be used to identify and message patients to improve contraception management. Follow-up phone calls for non-responders, which could be conducted by trained staff rather than physicians, further increases successful communication.
To cite this abstract in AMA style:
Bean M, Coburn B, Riley T, Breed E, García-González C, Gupta A, Kotzin J, Mayer A, Oghene J, Romich E, George M, Dayno R, Sandorfi N, Thomas P, Shahane A. A Quality Improvement Project to Improve Contraception Management in Patients with Systemic Lupus Erythematosus on Teratogenic Medications [abstract]. Arthritis Rheumatol. 2024; 76 (suppl 9). https://acrabstracts.org/abstract/a-quality-improvement-project-to-improve-contraception-management-in-patients-with-systemic-lupus-erythematosus-on-teratogenic-medications/. Accessed .« Back to ACR Convergence 2024
ACR Meeting Abstracts - https://acrabstracts.org/abstract/a-quality-improvement-project-to-improve-contraception-management-in-patients-with-systemic-lupus-erythematosus-on-teratogenic-medications/