Session Information
Session Type: Poster Session C
Session Time: 10:30AM-12:30PM
Background/Purpose: Macrophage Activation Syndrome (MAS) is a pathologic condition of immune hyperactivation, which occurs in 10-30% of cases of Still’s Disease (SD), the spectrum of systemic Juvenile Idiopathic Arthritis (sJIA) and Adult-onset Still’s Disease, and represents one of its major cause of mortality. Although treatment of sJIA has become highly effective with targeted biologicals, such as IL-1 or IL-6 blockade, long term follow-up studies show no reduction in the incidence of MAS1. The immunological connections and underlying mechanisms between MAS and SD are still incompletely understood. Recently, a population of highly proliferative CD38+HLA-DR+CD8+T-cells has been identified to be expanded and to act as a major source of IFN-g in MAS patients2. Although these cells have been thoroughly characterized in MAS, only limited data are available about these cells during the disease course of SD and about the mechanism which leads to their expansion. The aim of this research was to evaluate this subset of CD8+T-cells longitudinally in patients with sJIA at different stages of disease. In addition, we sought to identify factors which contribute to the increase of this cell population.
Methods: The percentage of CD38+HLA-DR+CD8+T-cells in peripheral blood mononuclear cells (PBMC) in paired samples of patients with active sJIA (n=7), inactive sJIA (n=6), MAS (n=3) and healthy donors (HD) (n=5), was quantified using flowcytometry. Then, we cultured PBMC and magnetic-isolated CD8+T-cells of HD with different stimulants (IL-1, IL-6, IL-18, IFNα and IL-15, toll-like-receptor 3,7,9 agonists). The percentage of CD38+HLA-DR+CD8+T-cell induction was evaluated over a duration of 7 days.
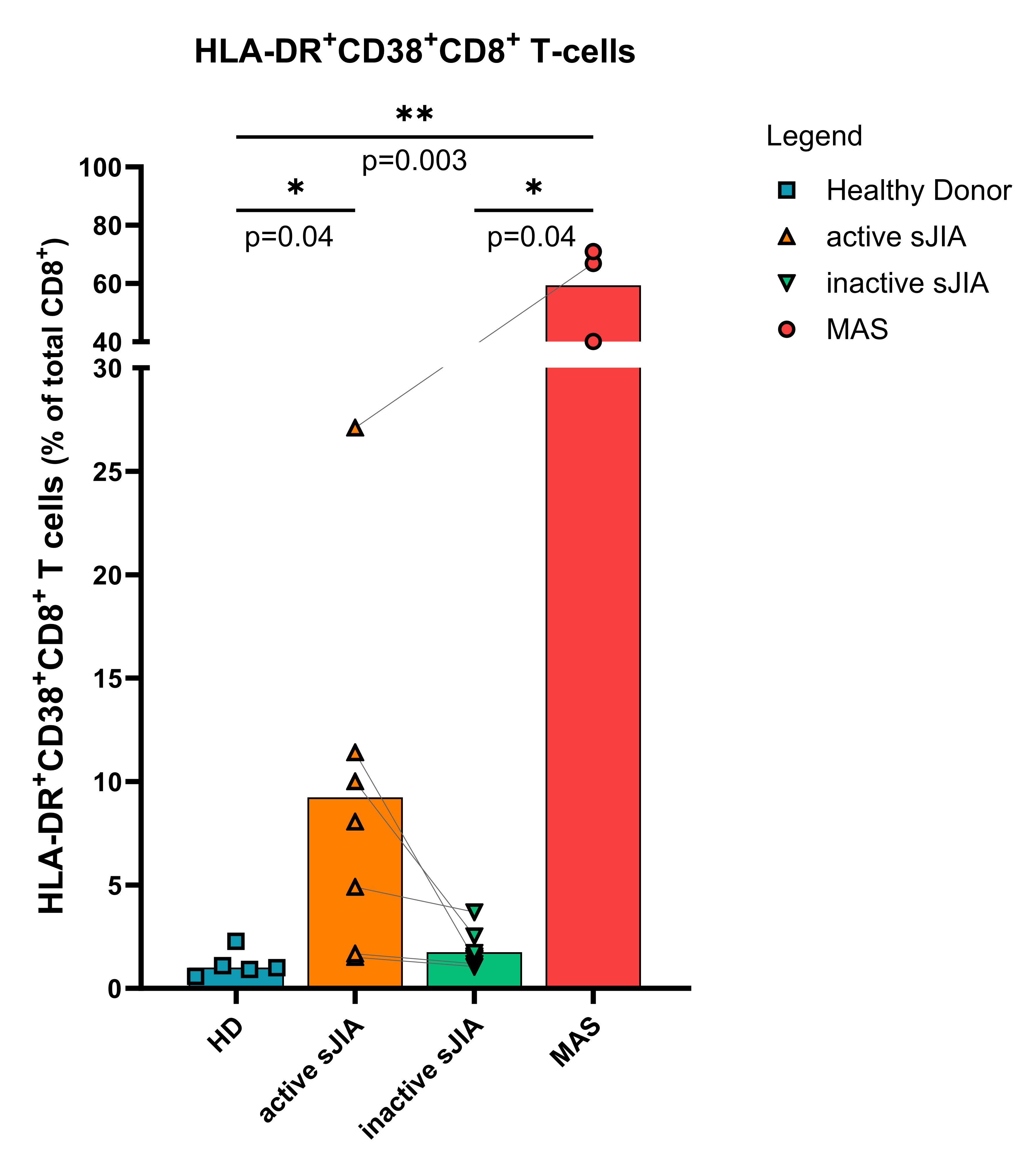
Results: The percentage of CD38+HLA-DR+CD8+T-cells was significantly increased in patients with active SD compared to HD (p=0.04) and increased, although not significantly, compared to patients in remission (figure 1). Similarly to what was previously reported, we observed a significant increase of CD38+HLA-DR+CD8+T-cells in patients with MAS compared to HD (p=0.003). Our in vitro data confirmed a prior observation that stimulation with IFNα and IL-15 induces these activated CD8+cells after a short-term stimulation of 48 hours2. At day 7 (representing long term stimulation), IL-18 was also able to induce high percentage of CD38+HLA-DR+CD8+T-cells. The induction of CD38+HLA-DR+CD8+T-cells by INFα and IL-15, and IL-18 over time was comparable between PBMCs and isolated CD8+cells, suggesting a possible direct role of IL-18 stimulation on CD8+cells.
Conclusion: We observed that CD38+HLA-DR+CD8+T-cells, which have specifically been associated with MAS, are also increased in SD patients with active disease (although to a lower extent), representing a potential biological connection between these two disorders. Moreover, 7-day in vitro stimulation with IL-18, known to be elevated in the plasma of sJIA patients and even higher in MAS patients, significantly increased the percentages of this active CD8+cell population. These data suggest that high IL-18 levels in sJIA and MAS might contribute in the expansion of this cell population, and therefore, in the susceptibility for this potentially fatal complication.
1Erkens, 2024
2Huang, 2023
To cite this abstract in AMA style:
Rogani G, Erkens R, Putmans M, Scholman R, van Loosdregt J, Vastert S. A Potential Role of Longstanding IL-18 Stimulation in the Susceptibility for Macrophage Activation Syndrome in Patients with Systemic Juvenile Idiopathic Arthritis [abstract]. Arthritis Rheumatol. 2024; 76 (suppl 9). https://acrabstracts.org/abstract/a-potential-role-of-longstanding-il-18-stimulation-in-the-susceptibility-for-macrophage-activation-syndrome-in-patients-with-systemic-juvenile-idiopathic-arthritis/. Accessed .« Back to ACR Convergence 2024
ACR Meeting Abstracts - https://acrabstracts.org/abstract/a-potential-role-of-longstanding-il-18-stimulation-in-the-susceptibility-for-macrophage-activation-syndrome-in-patients-with-systemic-juvenile-idiopathic-arthritis/