Session Information
Session Type: Poster Session B
Session Time: 10:30AM-12:30PM
Background/Purpose: Two randomized controlled phase 2 trials in patients with active Sjogren’s disease (SjD) were recently completed, the LOUiSSe trial assessing the Bruton Tyrosine kinase inhibitor remibrutinib (LOU064; NCT04035668; Dörner et al (1), and the TwinSS trial assessing the fully human monoclonal antibody against CD40, iscalimab (CFZ533; NCT03905525). Both trials met their respective primary endpoints using the validated European Sjogren’s Syndrome Disease Activity Index (ESSDAI). Here we report a post-hoc analysis of the trials to explore performance of the newly proposed composite endpoint STAR.
Methods: Participants in both trials fulfilled the EULAR/ACR2016 classification criteria, were anti-Ro60-positive and had residual stimulated whole salivary flow. In the TwinSS trial, 173 patients were assigned to cohort 1 (ESSDAI ≥5 and European Sjogren’s Syndrome Patient Reported Index [ESSPRI] ≥5) and 100 to cohort 2 (ESSDAI < 5, ESSPRI (fatigue or dryness) ≥5. In the LOUiSSe trial, 73 patients were randomized in a 1:1:1 ratio to one of three treatment groups: remibrutinib 100 mg once per day or 100 mg twice per day, or placebo. In both trials, the primary endpoint was change from baseline in ESSDAI at week 24. STAR is a composite endpoint comprised of clinical ESSDAI (ClinESSDAI; includes 10 physician reported domains and one laboratory domain), ESSPRI (3 patients reported items), lachrymal gland function measured by Schirmer’s test; salivary gland function measured as stimulated salivary flow by drole collection, and a biologic domain. All available w24 data (the primary endpoint) were analyzed for the current investigation of STAR. The derivation codes of the STAR endpoint were developed and provided by NECESSITY IMI2 consortium (2). Non-responder imputation was applied to handle the missing data.
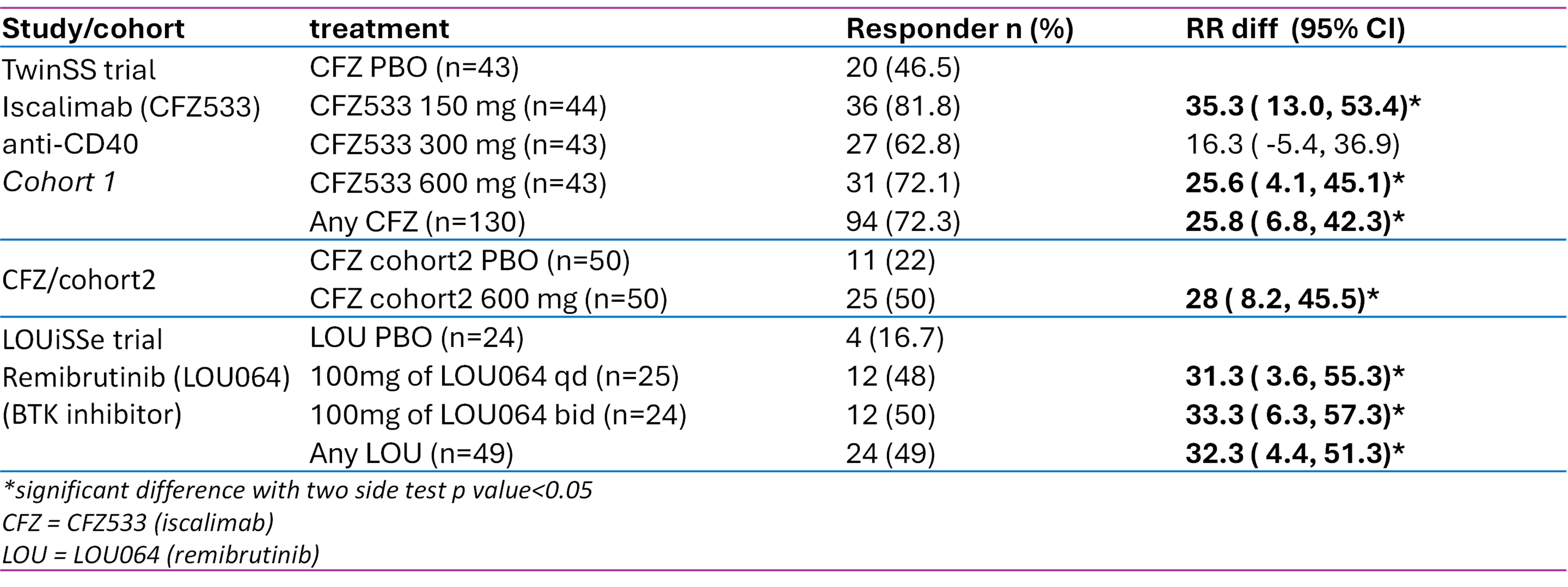
Results: Pooled across the active arms, the week 24 ESSDAI response rates in LOUiSSe were 29.2% on placebo (Pbo) versus (vs) 53.1% on active treatment, and in TwinSS cohort one 62.8% on Pbo vs 74.6% on active; The pooled ESSPRI response rates in LOUiSSe were 45.8% on Pbo versus 38.8% on active; in TwinSS cohort one, 58.1% on Pbo vs 63.1% on active treatment. For TwinSS cohort 2 (one active arm), ESSPRI response rates were 54.0% on Pbo vs 64.0% on active treatment. The week 24 STAR responder analysis revealed meaningful differences between the control arm and actively treated patients in both trials, with responder rate differences between active and placebo arm ranging from 26% to 35%, respectively. Notably, STAR also showed good differentiation of the placebo group from the group of patients treated with investigational drug in Cohort 2 of the TwinSS trial (Figure 1).
Conclusion: Our post-hoc exploratory analysis of two recent interventional phase 2 randomized controlled trials suggests utility of STAR as a single composite outcome measure for SjD studies. Encompassing all key disease features, STAR appears to perform well in major subsets of patients with SjD including a subgroup with high symptom burden and low systemic activity. STAR yields a comparatively low placebo response rate, and good differentiation between active and control arms.
To cite this abstract in AMA style:
Hueber W, Seror R, Porcher R, Baron G, He L, Luo W, Mariette X, Gergely P. A Post-hoc Analysis of Two Phase 2 Randomized Controlled Trials in Patients with Active Sjogren’s Disease Exploring the Novel Composite Endpoint Sjögren’s Tool for Assessing Response (STAR) [abstract]. Arthritis Rheumatol. 2024; 76 (suppl 9). https://acrabstracts.org/abstract/a-post-hoc-analysis-of-two-phase-2-randomized-controlled-trials-in-patients-with-active-sjogrens-disease-exploring-the-novel-composite-endpoint-sjogrens-tool-for-assessing-response/. Accessed .« Back to ACR Convergence 2024
ACR Meeting Abstracts - https://acrabstracts.org/abstract/a-post-hoc-analysis-of-two-phase-2-randomized-controlled-trials-in-patients-with-active-sjogrens-disease-exploring-the-novel-composite-endpoint-sjogrens-tool-for-assessing-response/