Session Information
Date: Tuesday, October 28, 2025
Title: (2227–2264) Rheumatoid Arthritis – Diagnosis, Manifestations, and Outcomes Poster III
Session Type: Poster Session C
Session Time: 10:30AM-12:30PM
Background/Purpose: RA is a heterogenous condition with variable outcomes. The identification of novel autoantibodies and autoantibody signatures could help us identify potential diagnostic and prognostic biomarkers which could guide treatment decisions. We carried out this pilot study using a high-throughput protein microarray platform to identify autoantibodies against native autoantigens in RA patients.
Methods: Sera from 10 RA and 10 gender-, age-, and ethnicity-matched healthy controls (HC) were screened for seroreactivity (IgG) to more than 1,600 disease-associated protein antigens in their native conformation. R package limma was used to perform a linear modeling analysis between comparison classes across all signals representing cognate antibody-antigen interactions. This analysis employs a moderated t-statistical method which calculates, for each antigen, the difference between two specified classes, and determines the significance of these differences by measure of fold changes and p-values .The significance threshold for identifying differential Abs (DAbs) was set at p < 0.05 and |fold-change| > 1.1. ROC analysis was performed between comparison classes across all antigens surpassing significance threshold during the above-described linear modeling analysis.
Results: 17 antibodies were identified to be differentially expressed (Figure A heatmap). Of note, higher levels of anti-TROVE2 IgG ( fold change of 1.97, p value = 4.09e-02, ROC AUC = 0.89) and anti-IRF5 IgG (fold change of 2.06, p value = 3.2e-03, ROC AUC = 1) were observed solely among RA patients, whereas higher levels of anti-HCLS1 IgG (fold change of 1.54, p value = 6.16e-03, ROC AUC 0.79) and anti-SPG20 IgG (fold change of 1.26, p value = 4.63e-03, ROC AUC 0.91) were nearly unique to HC (Figure B1-4).
Conclusion: A high-throughput protein microarray platform allows detection and quantification of autoantibodies which maybe of potential pathophysiological and prognostic significance in RA patients. TROVE2 is a well-known classical rheumatological autoantigen and has been shown to be a biomarker for predicting ADAbdevelopment and therapeutic response in adalimumab-treated patients. IRF5 is involved in signal transduction eliciting type I IFN expression. Additionally, upregulation of antibodies targeting HCLS1 (involved in leukopoiesis and leukocyte clonal deletion) and SPG20 (involved in lipid degradation and mitochondrial function) in HC suggests possible protection against RA by pathways incorporating those proteins, or indeed by the antibodies that target them. Our preliminary findings suggest a complex relationship between autoantibody signatures and RA.
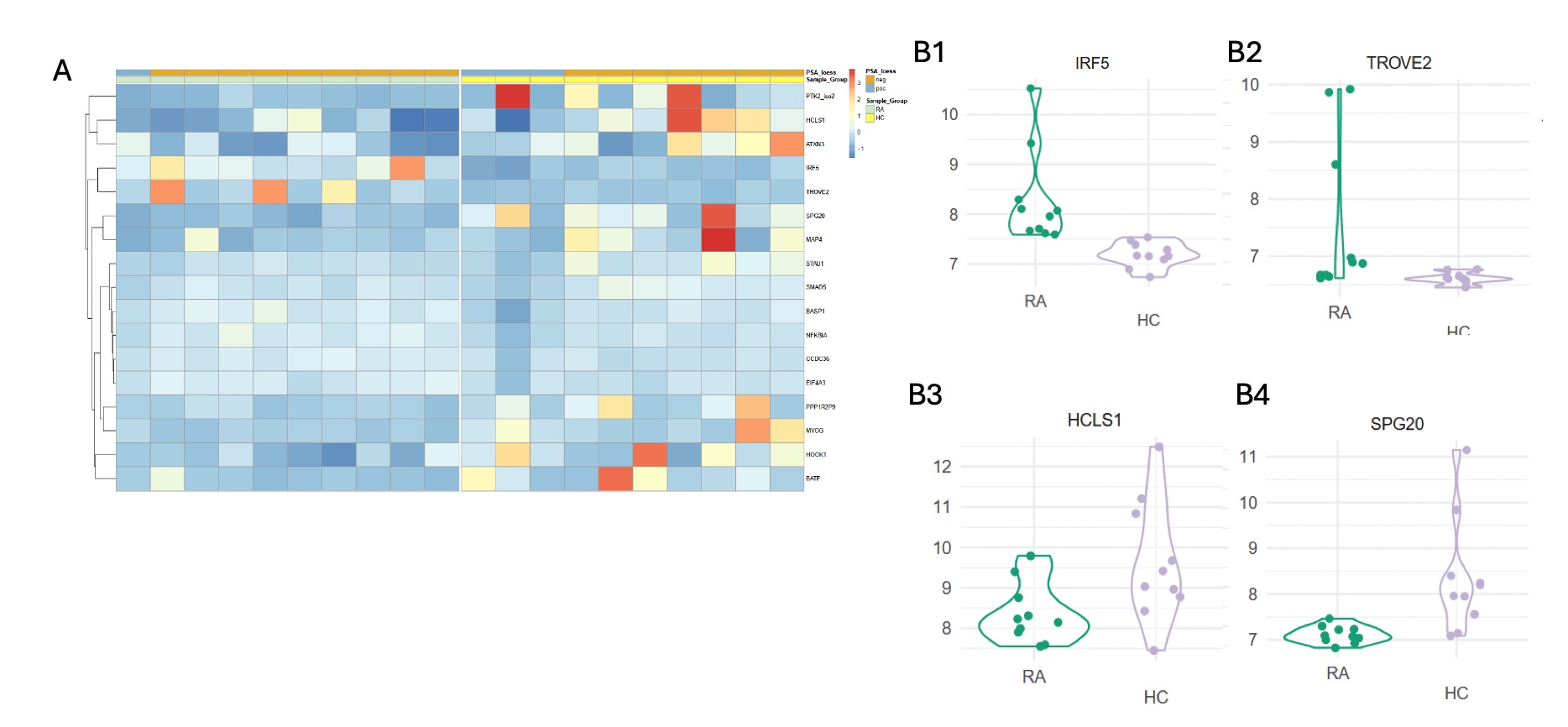 Figure: A = annotated heatmap of the signals for antigens surpassing significance threshold during linear modeling analysis, with both row and column clustering. B1-4 = violin plots of the autoantibodies of the most significance.
Figure: A = annotated heatmap of the signals for antigens surpassing significance threshold during linear modeling analysis, with both row and column clustering. B1-4 = violin plots of the autoantibodies of the most significance.
To cite this abstract in AMA style:
Ma M, Cheung P, koh e, peyper j, abylova b, Leong K. A Pilot Study to identify novel autoantibody biomarkers in Rheumatoid Arthritis (RA) patients using Immunome Protein Array [abstract]. Arthritis Rheumatol. 2025; 77 (suppl 9). https://acrabstracts.org/abstract/a-pilot-study-to-identify-novel-autoantibody-biomarkers-in-rheumatoid-arthritis-ra-patients-using-immunome-protein-array/. Accessed .« Back to ACR Convergence 2025
ACR Meeting Abstracts - https://acrabstracts.org/abstract/a-pilot-study-to-identify-novel-autoantibody-biomarkers-in-rheumatoid-arthritis-ra-patients-using-immunome-protein-array/
