Session Information
Session Type: Abstract Submissions (ACR)
Background/Purpose: Interleukin 10 (IL10) is an anti-inflammatory cytokine potentially efficacious for RA. F8-IL10 is a fusion protein in which the cytokine is fused with the antibody F8 specific to the alternatively-spliced EDA domain of fibronectin, a marker of angiogenesis. The conjugation of IL10 to the antibody F8 allows the selective delivery and accumulation of the cytokine to sites of inflammation, therefore increasing the therapeutic index. In mouse models of collagen-induced arthritis, F8-IL10 was able to selectively localize at sites of inflammation and showed a clear therapeutic activity by drastically reducing paw swelling when combined with MTX. A Phase Ib clinical trial is now on-going involving the administration of F8-IL10 in combination with MTX in patients with RA who have previously failed at least one TNF blocker. Objectives of the study are to establish the maximum tolerated dose of the combined treatment (F8-IL10 + MTX), to study safety and tolerability, to obtain preliminary information on efficacy and to assess the pharmacokinetic behavior of the drug. Here, we report the results obtained in 5 patients who have already completed the study. A sixth patient is under treatment at the time of writing.
Methods: Cohorts of 3-6 patients with active RA are assigned to receive escalating doses of F8-IL10 (6, 15, 30, 60 µg/kg respectively) in combination with 15mg of MTX. The treatment is given as once weekly sc injection for up to 8 weeks. Safety evaluation performed on days 1 through 28, including AEs, SAEs, and standard laboratory assessments, are used to determine the dose limiting toxicity. Response is assessed after 4 and 8 weeks of treatment according to ACR and DAS28 criteria. The pharmacokinetic profile and formation of human anti-fusion protein antibodies are measured using standard methods.
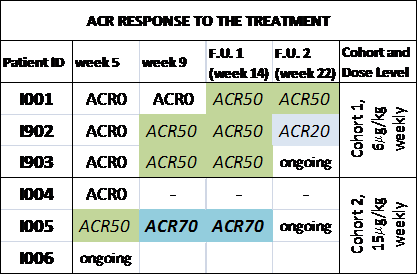
Results: All three patients enrolled in the first cohort (6 µg/kg weekly of F8-IL10) achieved an ACR 50 response at more than one evaluation time point. In cohort 2 (15 µg/kg weekly of F8-IL10), patient I005 even resulted in ACR 70 response whereas patient I004 did not reach ACR20, however a moderate EULAR response was seen and treatment stopped after only 4 weeks. ACR responses are summarized in the attached table. DAS 28 significantly improved in all patients but to a lesser extent in pt I004. An excellent tolerability of F8-IL10, at the doses used, was observed in all treated patients, as of now no grade ≥2 adverse drug reactions have been reported.
Conclusion: The promising safety data regarding the clinical use of F8-IL10, together with preliminary positive signs of activity may be favored by the targeted delivery of IL10 to the site of inflammation. These results also warrant future developments of the product in randomized clinical trials, which are currently in planning. An update of clinical data will be presented at the ACR meeting.
Disclosure:
M. Galeazzi,
None;
C. Baldi,
None;
E. Prisco,
None;
M. Bardelli,
None;
D. Neri,
Philogen,
4;
L. Giovannoni,
Philogen,
3;
E. Selvi,
None;
R. Caporali,
None.
« Back to 2012 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/a-phase-ib-clinical-trial-with-f8-il10-an-anti-inflammatory-immunocytokine-for-the-treatment-of-rheumatoid-arthritis-ra-used-in-combination-with-methotrexate-mtx/