Session Information
Session Type: Poster Session C
Session Time: 10:30AM-12:30PM
Background/Purpose: In SLE, ANAs can promote pathogenesis by forming immune complexes (ICs) that activate complement. While antibodies to DNA (anti-DNA) are known to be associated with complement, the role of other ANAs in activating complement is less clear. To elucidate better serological biomarkers in the context of novel therapies to decrease immunoglobulin levels or B-cells, we modeled the relationship between autoantibodies (anti-DNA, other ANAs, anti-C1q) and complement.
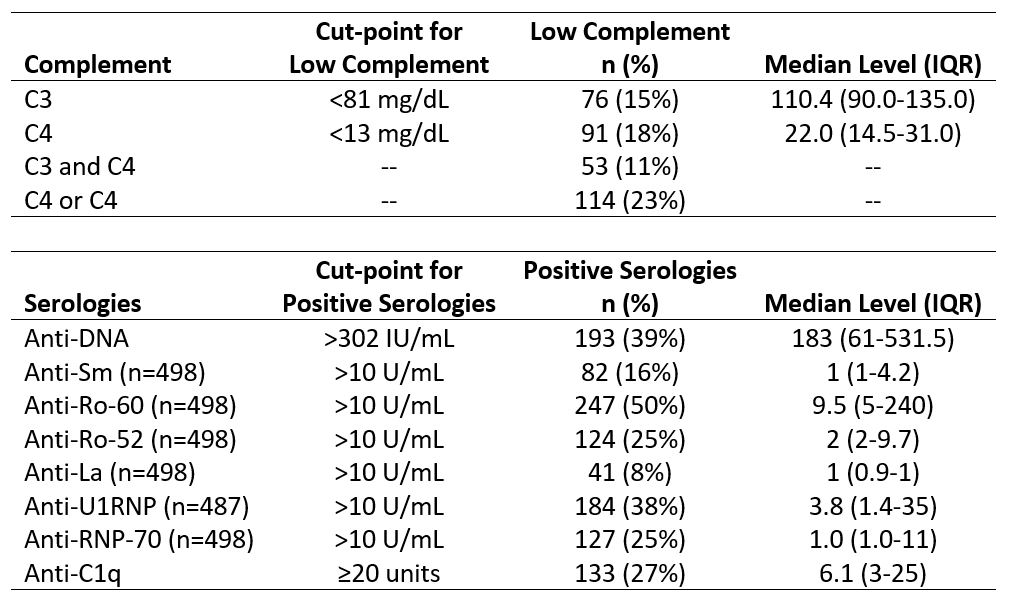
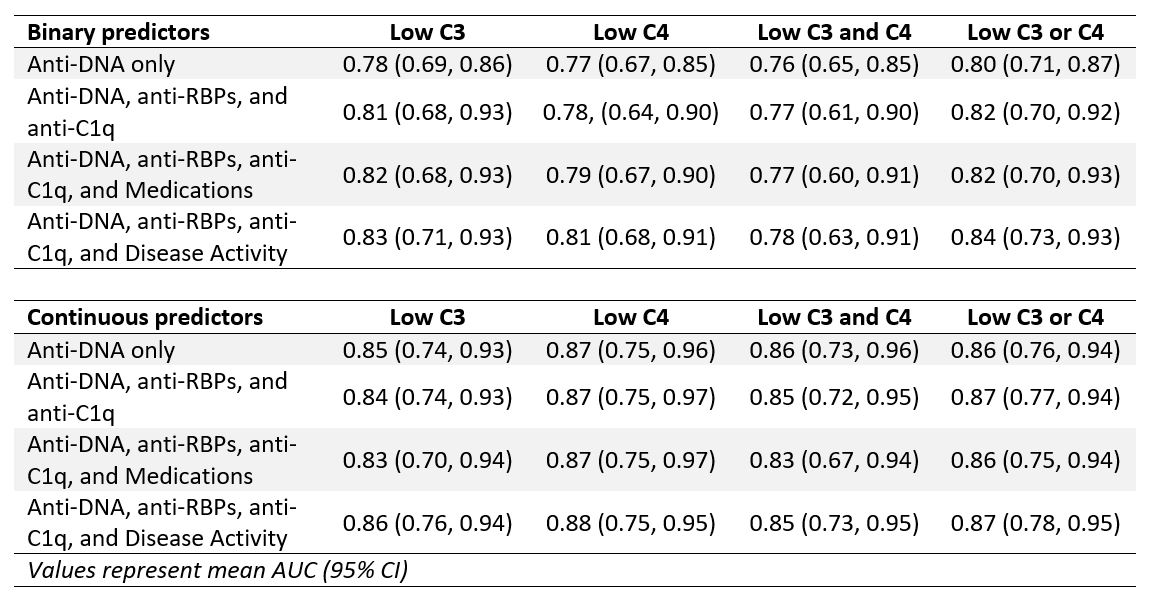
Methods: Adult SLE patients (SLICC or ACR/EULAR criteria) were enrolled during routine clinic visits from June 2020 to March 2024. At each visit, treating rheumatologists scored the PGA and SLEDAI, medications were recorded, and autoantibodies were measured. Autoantibodies including ANA, anti-DNA, anti-RNA-binding proteins (RBPs), and anti-C1q were measured by ELISA. Complement activation was defined as (1) low C3, (2) low C4, (3) low C3 and low C4, and (4) low C3 or low C4. Potential predictors of complement activation were modeled in 4 steps: (1) anti-DNA; (2) anti-DNA, anti-RBPs (Ro-52, Ro-60, Sm, La, U1RNP, RNP-70), and anti-C1q; (3) anti-DNA, anti-RBPs, anti-C1q, and medications; and (4) anti-DNA, anti-RBPs, anti-C1q, and disease activity. To identify linear and possible nonlinear relationships between predictors and complement activation, we considered both generalized linear models (GLMs; specifically, logistic regression with LASSO regularization) and neural networks, each with both binary and continuous predictors. Models were trained and tuned on 80% of patients and evaluated on the remaining 20%.
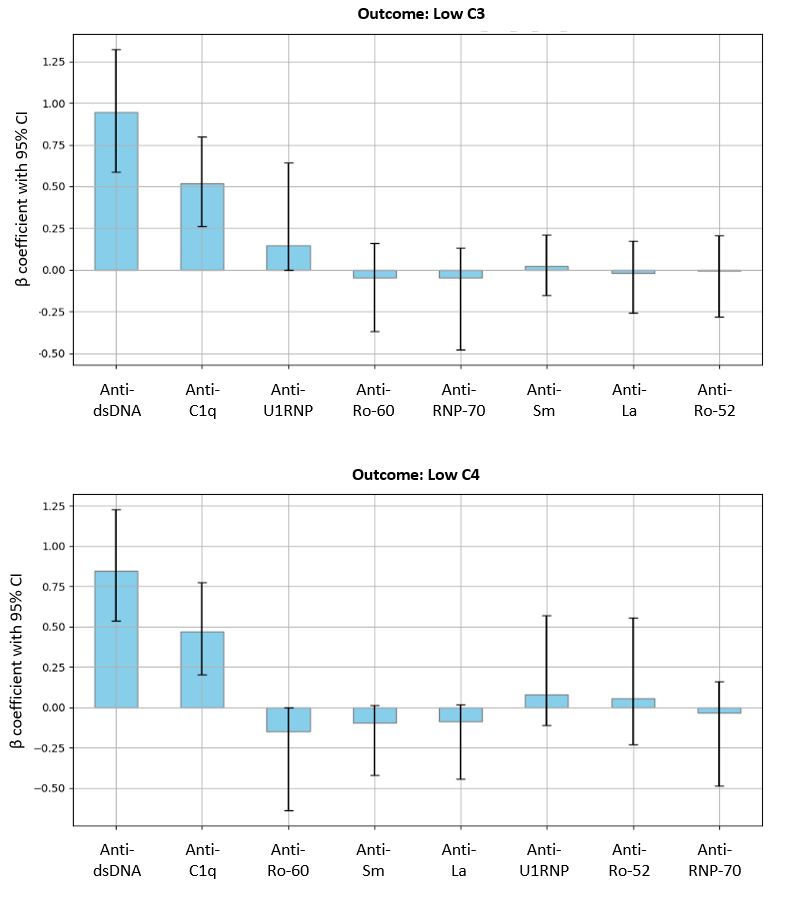
Results: The study included 500 visits in 242 patients (mean age 42 years; mean disease duration 13 years; 89% female; 59% Black, 29% White; 6% Hispanic). Almost one-quarter of visits had low C3 or C4; anti-DNA was positive at 39% of visits (Table 1). In models with binary predictors, the presence of anti-DNA most accurately predicted complement activation (AUC: 0.76-0.80; Table 2); model performance improved with the inclusion of anti-RBPs and anti-C1q (AUC: 0.77-0.82). The inclusion of medications or disease activity led to limited improvement in model performance. Continuous predictors performed better than binary predictors. Continuous anti-DNA level alone predicted low complement (AUC: 0.85-0.87; Table 2), as well as anti-DNA combined with anti-RBPs and anti-C1q (AUC: 0.84-0.87). Across outcomes of complement activation, anti-DNA and anti-C1q were consistently associated with low complement. Of medication and disease activity variables, active lupus nephritis most consistently predicted low complement in both binary and continuous models.
Conclusion: These results support the important role of anti-DNA antibodies in complement activation as reflected in levels of C3 and/or C4; the effects of other ANAs in the model were less marked, perhaps reflecting a more limited ability to form ICs that activate complement. The association of anti-C1q with low C3 and/or C4 is consistent with a role in activating complement and suggests the value of assaying anti-C1q in studies on therapies that can impact autoantibody levels.
To cite this abstract in AMA style:
Pisetsky D, Zhao Y, Engelhard M, Eudy A, Tedeschi P, Verdone A, Clowse M, Criscione-Schreiber L, Doss J, Maheswaranathan M, Sadun R, Sun K, Rogers J. A Novel Modeling Approach to Elucidate the Role of Autoantibodies in Complement Activation in SLE [abstract]. Arthritis Rheumatol. 2024; 76 (suppl 9). https://acrabstracts.org/abstract/a-novel-modeling-approach-to-elucidate-the-role-of-autoantibodies-in-complement-activation-in-sle/. Accessed .« Back to ACR Convergence 2024
ACR Meeting Abstracts - https://acrabstracts.org/abstract/a-novel-modeling-approach-to-elucidate-the-role-of-autoantibodies-in-complement-activation-in-sle/