Session Information
Date: Sunday, November 12, 2023
Title: Abstracts: B Cell Biology & Targets in Autoimmune & Inflammatory Disease
Session Type: Abstract Session
Session Time: 2:00PM-3:30PM
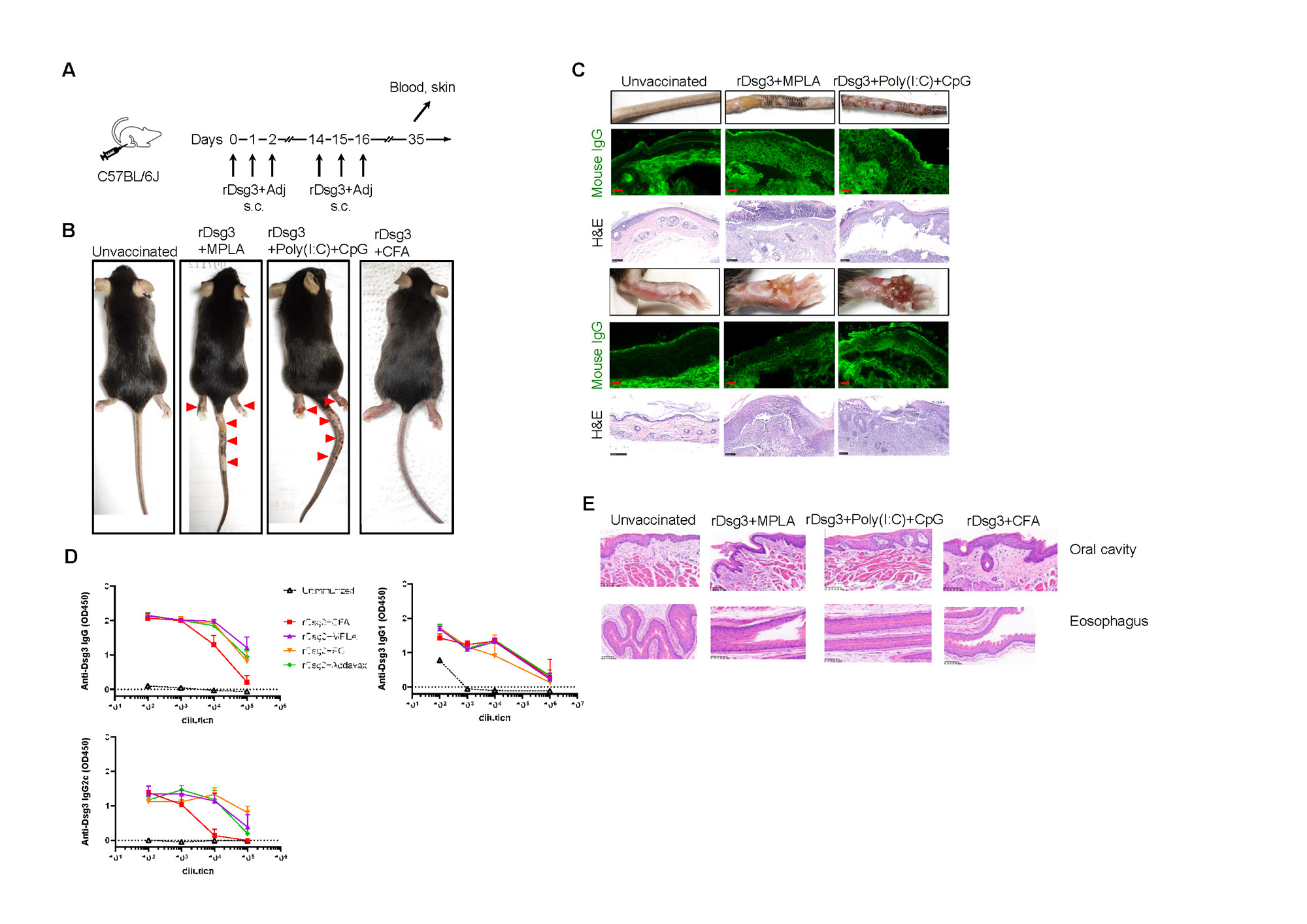
Background/Purpose: Pemphigus vulgaris (PV) is an autoimmune bullous skin disease mediated by desmoglein-3 (Dsg3) specific auto-antibody present in skin and mucosae. The binding of pathogenic antibody to Dsg3, which are cell-cell adhesion molecules found in desmosome, induces loss of adhesion between keratinocyte in epidermis and blister formation, resulting in painful erosion in PV patient and are even fatal when severe. Animal model plays vital role in developing safe and effective therapies for PV. However, the PV adult animal model is quite limited, with the adoptive transfer mouse model being the most widely-used, which requires both Dsg3 knock-out mice and immune-deficient mice as donor and recipient mice, respectively. In this study, we aimed to develop a novel active adult PV model to overcome some of the limitations of current animal models.
Methods: The recombinant Dsg3 (rDsg3) protein vaccines were prepared by mixing rDsg3 and different candidate immune adjuvants for induction of active PV model. Wild-type C57BL/6J mouse was immunized by subcutaneous injection in the footpad through a prime/boost regime. The gross phenotype was monitored and plasma was prepared periodically post immunization. Enzyme-linked immunosorbent assay (ELISA) was conducted for measurement of anti-Dsg3 antibody levels. Passive neonatal mouse model was used to determine the pathogenicity of plasma from PV model mouse. Skin tissues were harvest and formalin-fixed, paraffin-embedded for histological examination, or snap-frozen in liquid nitrogen and embedded in OCT for direct immunofluorescence (IF) to detect the deposition of auto-antibodies.
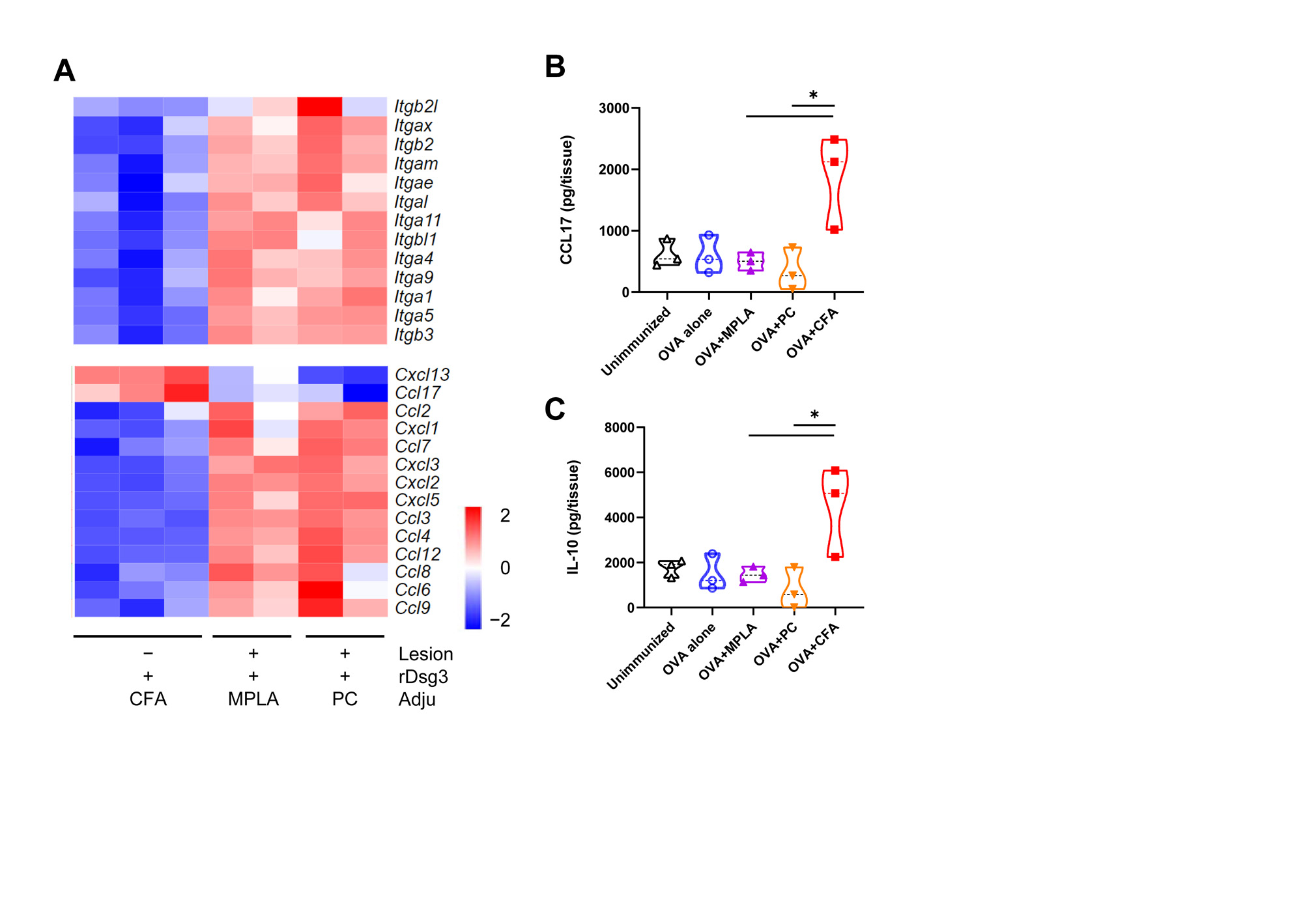
Results: We found that immunization with rDsg3 adjuvanted with single or a combination of TLR ligands could induce blister formation, skin erosion and hemorrhage in the footpad and tail. H&E staining indicated suprabasilar blister with acantholysis in skin tissues. Direct IF showed deposition of IgG between keratinocyte in the epidermis of PV model mice. A significant elevation of anti-Dsg3 level was observed in plasma of PV model mice as compared with unvaccinated mice measured by ELISA. We also found that completeFreund’s adjuvant could not facilitate the breach of tolerance against self-desmoglein 3, as indicated by gross phenotype and histological examination. Bulk RNA-seq suggested Treg-attractant chemokine Ccl17 was down-regulated by TLR ligand, which was confirmed by ELISA at the protein level, indicating the reduced recruitment of regulatory T cell into the skin lesion and uncontrolled autoimmunity against Dsg3 when TLR ligands was applied.
Conclusion: A novel active pemphigus vulgaris mouse model was established by immunization wild-type C57BL/6J mouse with adjuvanted recombinant desmoglein 3 protein vaccine, which closely mimic clinical symptoms of mucocutaneous type of PV patients. This model could compensate for current PV models with no need of either Dsg3 knock-out mice or immune-deficient mice, and greatly promote the development of antigen-specific therapies for PV.
To cite this abstract in AMA style:
Gao C, Wang Z, Lu Q. A Novel Active Pemphigus Vulgaris Mouse Model Induced by Immunization with TLR Ligand Adjuvanted Recombinant Desmoglein 3 Protein Vaccine [abstract]. Arthritis Rheumatol. 2023; 75 (suppl 9). https://acrabstracts.org/abstract/a-novel-active-pemphigus-vulgaris-mouse-model-induced-by-immunization-with-tlr-ligand-adjuvanted-recombinant-desmoglein-3-protein-vaccine/. Accessed .« Back to ACR Convergence 2023
ACR Meeting Abstracts - https://acrabstracts.org/abstract/a-novel-active-pemphigus-vulgaris-mouse-model-induced-by-immunization-with-tlr-ligand-adjuvanted-recombinant-desmoglein-3-protein-vaccine/