Session Information
Session Type: Abstract Session
Session Time: 10:00AM-10:15AM
Background/Purpose: Adults with dermatomyositis face a well-established 2–3-fold increased risk of major adverse cardiovascular events, driven by systemic inflammation, endothelial dysfunction, and treatment-related metabolic complications. In contrast, cardiovascular risk in juvenile dermatomyositis (JDM) remains poorly defined, despite a higher prevalence of clinical vasculopathy in children and emerging evidence that early, severe vasculopathic skin involvement is linked to cardiovascular dysfunction. Our previous work identified circulating calprotectin—a marker of neutrophil activation and NET formation—as a predictor of future cardiovascular disease (CVD) and a correlate of impaired HDL function in the general population. Notably, many JDM patients have elevated calprotectin levels. This study aimed to evaluate HDL function and early atherosclerotic risk in JDM and to investigate their potential association with calprotectin.
Methods: We assessed cholesterol efflux capacity (CEC, a measure of HDL function) in HDL-rich (apoB-depleted) plasma from 51 JDM patients and 24 pediatric controls without systemic autoimmune disease (Fig. 1A). We also measured plasma paraoxonase-1 (PON1) activity, an indicator of oxidative stress and HDL dysfunction linked to future CVD risk, and conducted a foam cell formation assay to evaluate plasma-driven fatty acid uptake, where greater uptake reflects a more pro-atherogenic profile.
Results: Compared to pediatric controls, JDM patients exhibited significantly impaired CEC and reduced PON1 activity (Fig. 1B–C), consistent with dysfunctional HDL. CEC was inversely correlated with fatty acid uptake in a foam cell assay (r = –0.39, p = 0.0045), indicating a shift toward a more pro-atherogenic state marked by increased lipid accumulation in macrophages. Among JDM patients, those with elevated circulating calprotectin had significantly lower CEC and PON1 activity (Fig. 2A–B), linking neutrophil activation and NET-associated inflammation to HDL dysfunction. Reduced CEC also correlated with clinical markers of disease activity, including elevated muscle enzymes such as creatine phosphokinase (CPK; r = –0.35, p = 0.012) and aspartate aminotransferase (AST; r = –0.28, p = 0.046), suggesting an association between active myositis and impaired vascular protection. Notably, JDM patients receiving immunomodulatory therapy (e.g., methotrexate) had significantly higher CEC than untreated patients (p = 0.04), raising the possibility that such treatments may help preserve or restore HDL function and vascular health beyond their anti-inflammatory effects.
Conclusion: JDM patients demonstrate marked HDL dysfunction that correlates with both heightened calprotectin levels and disease activity, underscoring a link between inflammation and early atherogenic risk. These findings support the importance of early cardiovascular surveillance and proactive management strategies in JDM patients, also highlighting the critical need to develop biomarkers of vascular health in children with JDM.
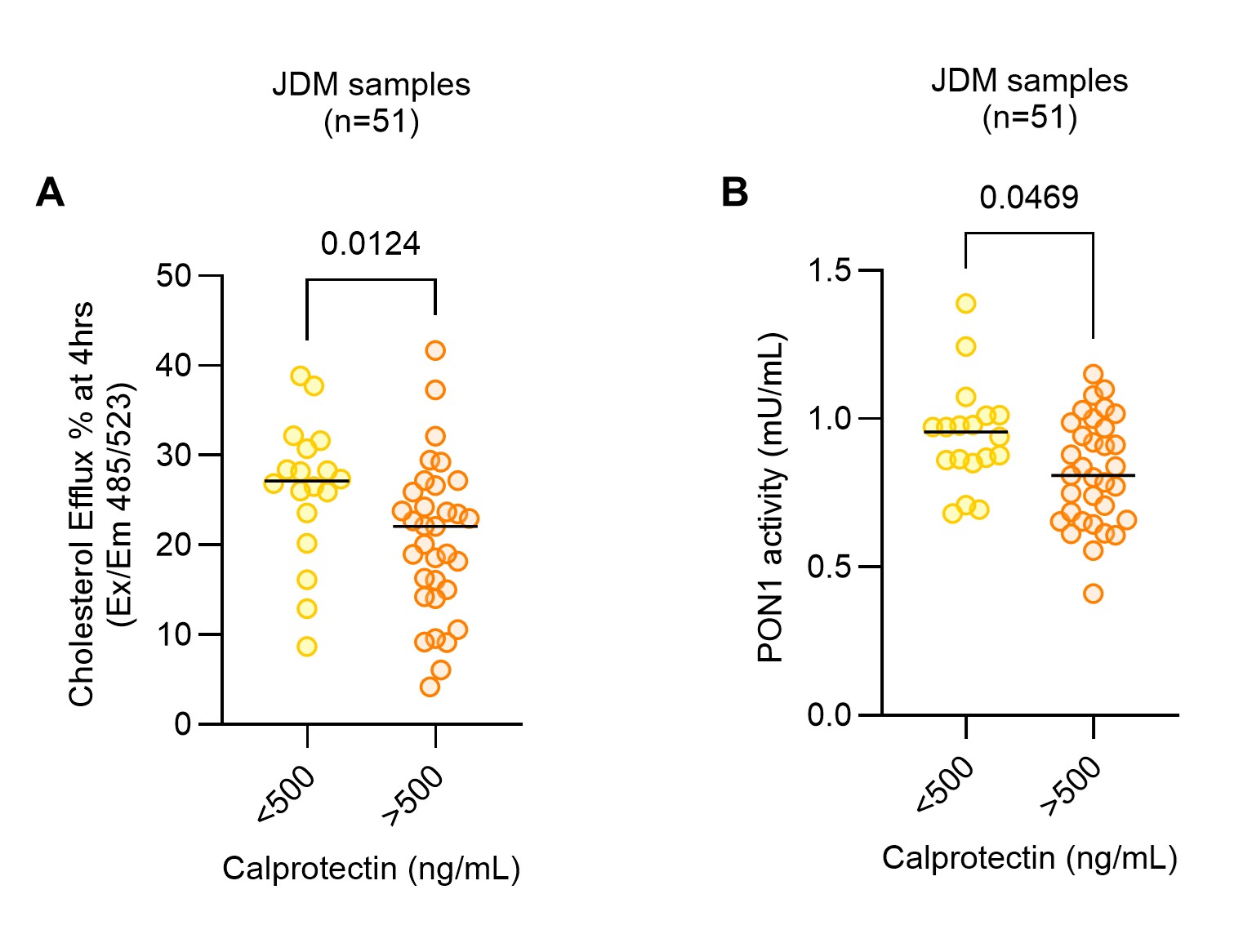 Figure 1: Impaired cholesterol efflux capacity (CEC) and reduced PON1 activity among JDM patients. A, Schematics of CEC assay. B, % CEC in JDM patients versus pediatric controls. C, Plasma PON1 Activity (mU/mL) in JDM patients compared to pediatric controls.
Figure 1: Impaired cholesterol efflux capacity (CEC) and reduced PON1 activity among JDM patients. A, Schematics of CEC assay. B, % CEC in JDM patients versus pediatric controls. C, Plasma PON1 Activity (mU/mL) in JDM patients compared to pediatric controls.
.jpg) Figure 2: JDM patients with elevated calprotectin levels exhibit impaired cholesterol efflux capacity and decreased PON1 activity. A, % CEC and B, Plasma PON1 Activity (mU/mL) in JDM patients with high calprotectin levels (>500 ng/mL) compared to those with low calprotectin levels ( < 500 ng/mL).
Figure 2: JDM patients with elevated calprotectin levels exhibit impaired cholesterol efflux capacity and decreased PON1 activity. A, % CEC and B, Plasma PON1 Activity (mU/mL) in JDM patients with high calprotectin levels (>500 ng/mL) compared to those with low calprotectin levels ( < 500 ng/mL).
To cite this abstract in AMA style:
Chong E, Sugur K, Matossian S, Kmetova K, Goudsmit C, Turnier J, Zuo Y. A New Link Between Calprotectin, Cholesterol Efflux Dysfunction, and Premature Atherosclerosis in Juvenile Dermatomyositis [abstract]. Arthritis Rheumatol. 2025; 77 (suppl 9). https://acrabstracts.org/abstract/a-new-link-between-calprotectin-cholesterol-efflux-dysfunction-and-premature-atherosclerosis-in-juvenile-dermatomyositis/. Accessed .« Back to ACR Convergence 2025
ACR Meeting Abstracts - https://acrabstracts.org/abstract/a-new-link-between-calprotectin-cholesterol-efflux-dysfunction-and-premature-atherosclerosis-in-juvenile-dermatomyositis/
