Session Information
Session Type: Poster Session A
Session Time: 9:00AM-11:00AM
Background/Purpose: The Janus Kinase inhibitors (JAKi) drugs are approved for use in adults with moderate to severe rheumatoid arthritis (RA). Real-world studies are necessary as they provide relevant data that complements the information provided by registration clinical trials.
Methods: Observational, retrospective, and multicenter study of a cohort of RA patients treated with a JAKi and followed up in n=14 Rheumatology Services, members of the RA Working Group (GT-ARCat) of the Catalan Society of Rheumatology (SCR). Data from patients who underwent JAKi treatment between May 2014 and November 2022 were included. Epidemiological and clinical variables were collected from baseline (S0) to week 52 (S52) of treatment. Analysis of the use of a first JAKi has been performed.
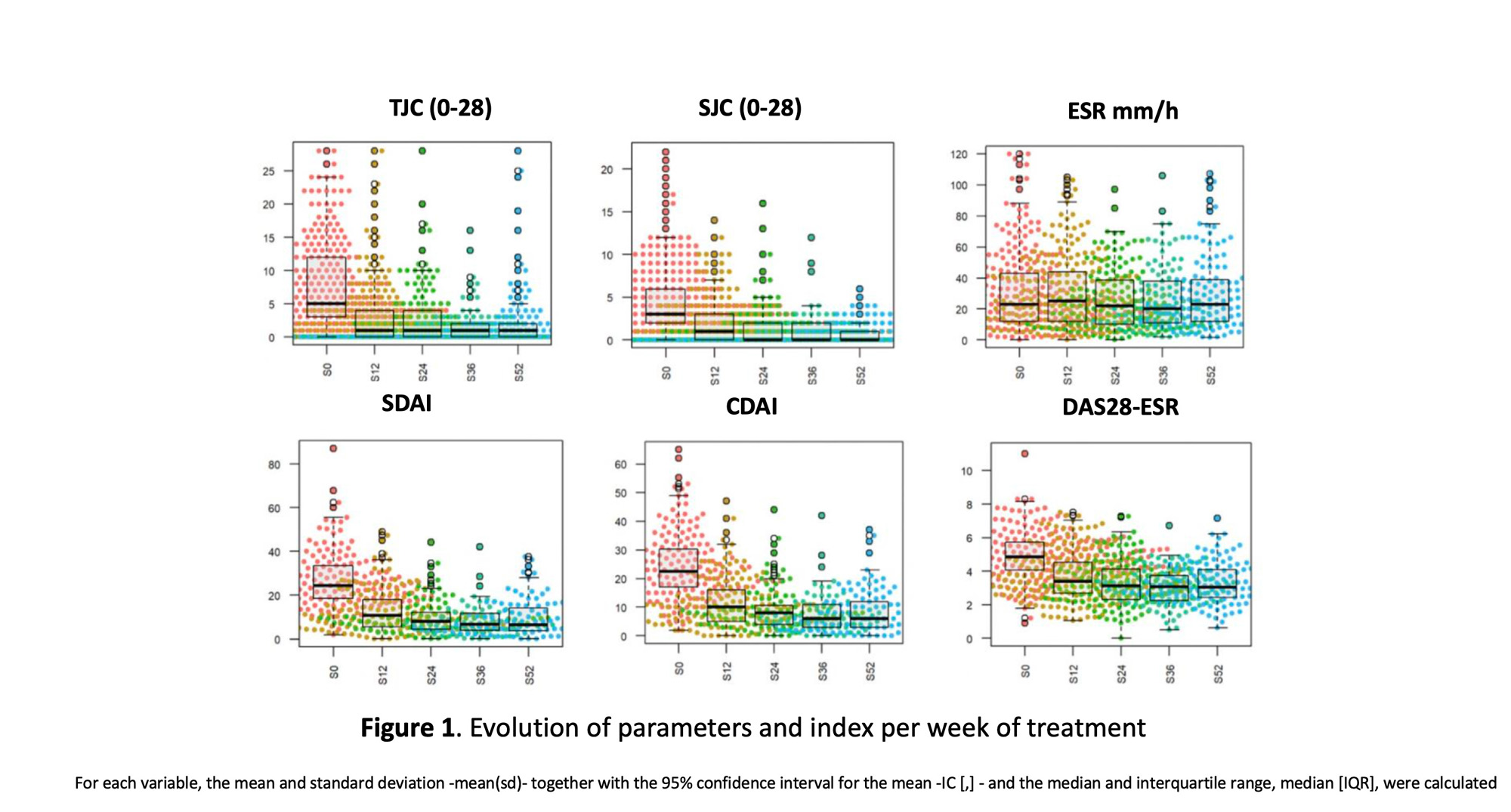
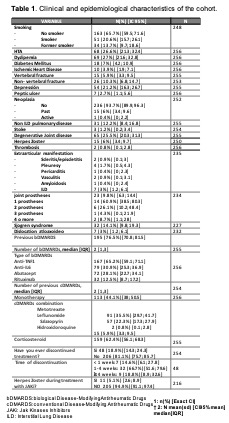
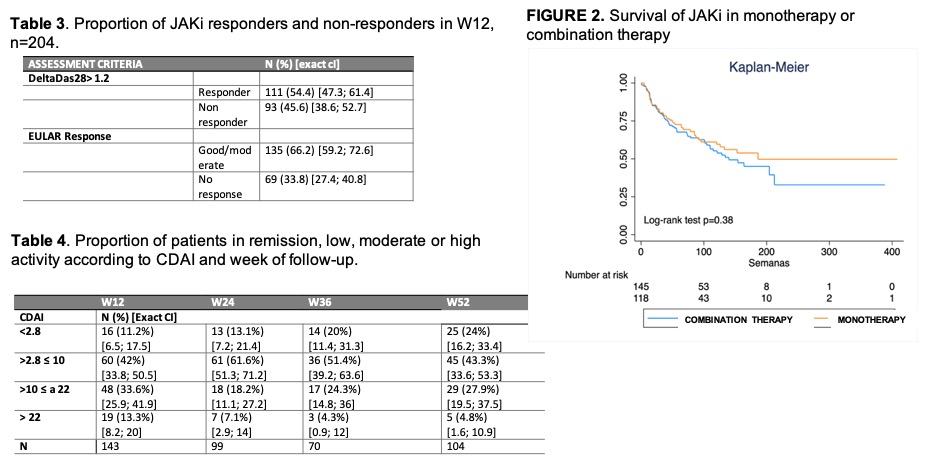
Results: A total of 256 patients (87% women) were included, with a mean age of 57.31 years and a mean time of disease evolution of 12.17 years. The cohort characteristics are detailed in Table 1. 53.5% of the cohort had received baricitinib, 41.8% tofacitinib and 4.7% upadacitinib. No statistically significant differences were observed in the epidemiological and clinical characteristics according to JAKi type. 65.6% of patients had at least one of the following characteristics (age ≥65 years, smoker or former smoker, HT, dyslipidemia, diabetes mellitus, ischemic heart disease, neoplasia, cerebral vascular accident or thrombosis). 36% had undergone ≥ 4 biological therapies (BT). During the follow-up period, treatment with a JAKi was withdrawn in 39.1% (n=100) of the cases included. The main reason for withdrawal was inefficacy in 57% (31% primary, 26% secondary), followed by 26% adverse effects and 17% other reasons. After treatment withdrawal with a JAKi, most patients (31.3%) were started on an anti-TNF drug, followed by an IL6 inhibitor (20.2%) or another JAKi (18.2%). Regarding the efficacy of JAKi treatment, a reduction in activity parameters and indices was observed throughout follow-up period as shown in Figure 1.The proportion of responders at follow-up weeks is detailed in Tables 2 and 3. The comparative analysis between responders and non-responders showed no statistically significant differences in descriptive and disease variables, except for number of previous bDMARDs (2 [RIQ 0.3] vs 2 [RIC 1.4], p=0.041, prior antiTNF use (57.8% vs 76.8%, p=0.011) and rituximab (7.4% vs 23.2%, p=0.003). A higher percentage of patients with DAS28 ≤2.6 was observed in the monotherapy group compared to the combinantion treatment (30.3% vs 17%, p=0.037). The median survival was 3.15 years and higher in monotherapy, although without statistically significant differences, Figure 2.
Conclusion: JAKi have been effective in different RA patient profiles while maintaining a good safety profile. In our cohort, although according to the latest recommendations, the use of JAKi would be limited in more than half of the patients, the withdrawal rate due to adverse effects has been low, although a longer follow-up time would be necessary.
To cite this abstract in AMA style:
Lopez-Lasanta M, Borrell H, Salles Lizarzaburu M, Ruiz-Esquide V, Roig D, Nack A, Pérez C, Bernardez J, García A, Rovira J, Cuervo A, Valls M, Garcia C, Minguez S, Morla R, Estrada P, Martinez M, Pitarch C, Busquets N, Park H, Gomez-Puerta J, Holgado S, Montala N, Sanmarti R, Mateo L, Diaz C, Corominas H, Salvador G. A Multicenter, Real-world Clinical Data Study of the Use of JAK Kinase Inhibitors in a Large Cohort of Patients with Rheumatoid Arthritis [abstract]. Arthritis Rheumatol. 2023; 75 (suppl 9). https://acrabstracts.org/abstract/a-multicenter-real-world-clinical-data-study-of-the-use-of-jak-kinase-inhibitors-in-a-large-cohort-of-patients-with-rheumatoid-arthritis/. Accessed .« Back to ACR Convergence 2023
ACR Meeting Abstracts - https://acrabstracts.org/abstract/a-multicenter-real-world-clinical-data-study-of-the-use-of-jak-kinase-inhibitors-in-a-large-cohort-of-patients-with-rheumatoid-arthritis/