Session Information
Date: Saturday, November 16, 2024
Title: Vasculitis – Non-ANCA-Associated & Related Disorders Poster I
Session Type: Poster Session A
Session Time: 10:30AM-12:30PM
Background/Purpose: Giant cell arteritis (GCA) is a granulomatous vasculitis affecting large vessels. The role of macrophages and vascular smooth muscle cells (VSMC) appears to be predominant in the pathophysiology of the disease. The standard treatment of GCA is based on glucocorticoids, which are remarkably effective, but do not prevent the development of vascular lesions and their consequences. Therefore, a better understanding of the pathophysiological mechanisms involved in GCA seems necessary to improve patient management. The epithelial growth factor receptor (EGFR) signaling pathway may play a role in some inflammatory diseases and may be involved in VSMC migration and proliferation. Heparin-binding epidermal growth factor (HBEGF) and amphiregulin (AREG) are the best-known EGFR ligands. Therefore, we aimed to investigate the role of the EGFR signaling pathway, especially its activation by AREG and/or HBEGF, in the pathophysiology of GCA.
Methods: Human material and cell lines were used in this study. Serum samples and temporal artery biopsies (TAB) were obtained from patients enrolled in the VASCO (VASculitis COhort) study
(NCT04413331)
, a prospective cohort of patients with systemic vasculitis. Two cell lines were also used: HAoSMC (human aortic VSMC) and THP-1, human monocytes.
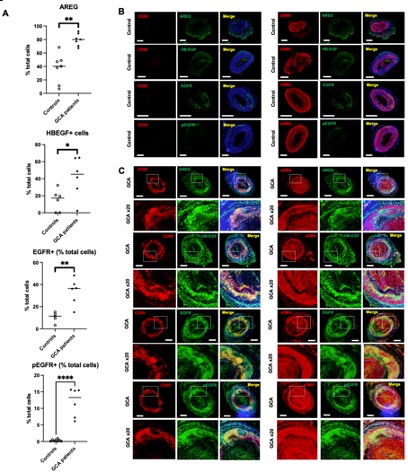
Results: Using multiplex immunohistochemistry and cell quantification techniques, we demonstrated that TAB from GCA patients expressed significantly higher levels of AREG, HBEGF, EGFR, and p-EGFR compared to controls (Figure 1A). Colocalization images showed that AREG, HBEGF and EGFR were predominantly expressed by macrophages and EGFR and p-EGFR by αSMA-positive cells (Figure 1B).
Using confocal fluorescence imagery, ELISA assays and transcriptomic, we found that AREG, HBEGF and EGFR were significantly higher in macrophages than in THP-1, especially in M1 pro-inflammatory macrophages. AREG and HBEGF levels were increased in the supernatant of M1 macrophages, and HBEGF in the supernatant of both M1 and M2 macrophages. Furthermore, stimulation of THP-1 cells with AREG or HBEGF induced the activation of the p38 MAPK pathway. Neither AREG nor HBEGF increased the production of pro-inflammatory cytokines by THP1 or macrophages.
Live cell imaging showed that AREG and HBEGF increased both VSMC proliferation and migration. Conversely, AG1478, an EGFR inhibitor, completely inhibited both VSMC migration and proliferation, suggesting a key role for the EGFR pathway in VSMC proliferation and migration. This role could be played by the activation of the MAPK pathway, as suggested by the Western blot technique performed in HAoSMC.
Conclusion: Our study shows that both AREG and HBEGF may play a role in the pathophysiology of GCA, especially in the remodeling phase of the disease. Further studies are needed to explore other EGFR ligands and to decipher the pathophysiological mechanisms between macrophages, VSMC and fibroblasts in GCA.
(A) Quantification of AREG, HB-EGF, EGFR and pEGFR-positive cells among temporal arteries from controls and GCA patients. Data show an increased expression of AREG, HB-EGF, EGFR and pEGFR in GCA compared to controls. P values were determined by the two-sided Kruskal-Wallis test, followed by Dunn’s post test for multiple group comparisons. *P <0.05; **P <0.01; ***P <0.001, ****P <0.0001. (B) Opal multiplex immunohistochemistry of temporal artery from a control using AREG, HB-EGF, EGFR, pEGFR, CD68 and αSMA antibodies. Illustrative pictures show a mild expression of AREG and HB-EGF in the adventitia of the temporal artery from controls, and the expression of EFGR but not pEGFR by αSMA-expressing cells in the media (Magnification x 2, scale bar 500 m). (C) Opal multiplex immunohistochemistry of temporal artery biopsy from a GCA patient using AREG, HB-EGF, EGFR, pEGFR, CD68 and αSMA antibodies. Illustrative pictures show a strong expression of AREG and HB-EGF by macrophages invading the media, with very few αSMA-positive cells expressing HB-EGF but not AREG, and both infiltrating macrophages and αSMA-positive cells in the media strongly expressed EGFR and pEGFR (Magnification x 2 and x 20, scale bar 500 m).
To cite this abstract in AMA style:
Chevalier K, Dionet L, Breillat P, Poux M, Dang J, Terris B, Bruneval P, Mouthon L, Lenoir O, Pierre-Louis T, Terrier B. A Macrophage-smooth Muscle Cell Axis Influences Vascular Remodeling Through Activation of the EGFR Pathway in Giant Cell Arteritis [abstract]. Arthritis Rheumatol. 2024; 76 (suppl 9). https://acrabstracts.org/abstract/a-macrophage-smooth-muscle-cell-axis-influences-vascular-remodeling-through-activation-of-the-egfr-pathway-in-giant-cell-arteritis/. Accessed .« Back to ACR Convergence 2024
ACR Meeting Abstracts - https://acrabstracts.org/abstract/a-macrophage-smooth-muscle-cell-axis-influences-vascular-remodeling-through-activation-of-the-egfr-pathway-in-giant-cell-arteritis/