Session Information
Date: Monday, November 14, 2022
Title: Metabolic and Crystal Arthropathies – Basic and Clinical Science Poster
Session Type: Poster Session D
Session Time: 1:00PM-3:00PM
Background/Purpose: ABP-671, a novel selective and potent URAT1 inhibitor reduces reabsorption of uric acid (UA) at the renal proximal tubule, and significantly decreases serum uric acid (sUA) levels in gout or hyperuricemia patients.
Methods: In this randomized, double-blind, placebo-controlled, ascending dose Phase 2a study, 59 patients with gout and 1 with hyperuricemia were enrolled in successive ascending dose groups of 2 mg, 4 mg, or 8 mg daily (20/group). Each dose group was subdivided into QD and BID cohorts (10:10) and received ABP-671 oral tablets or placebo in a 4:1 ratio. To moderate rapid increases in urinary uric acid, the dose of study drug was increased gradually in run-in periods of 1 – 3 weeks (1 week for Group 1, 2 weeks for Group 2, and 3 weeks for Group 3). Safety labs, sUA, serum creatinine, adverse events, vital signs, and EKGs were obtained at each visit.
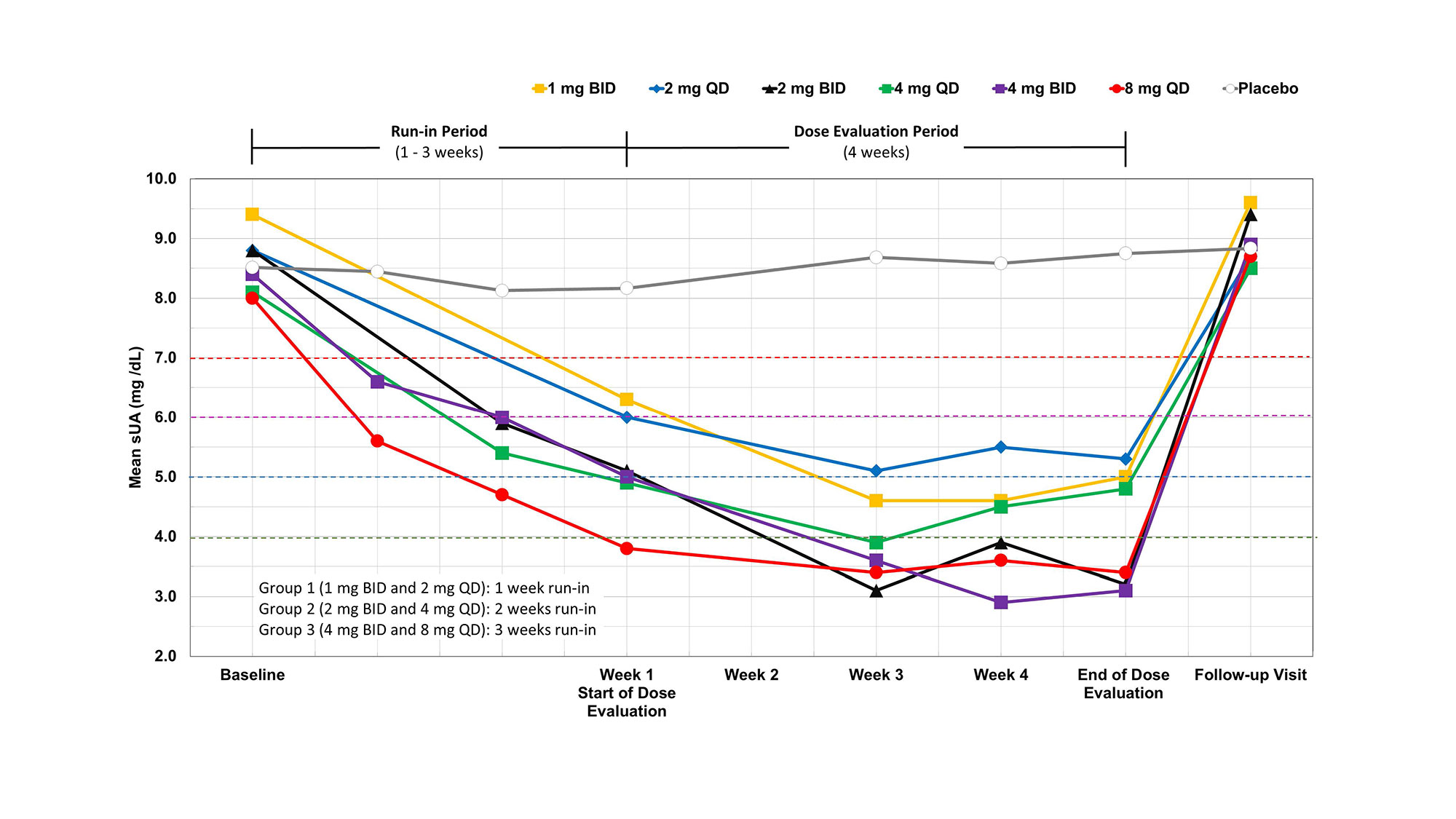
Results: Cohort mean and median sUA decreased rapidly during the run-in period for each of the 6 cohorts and further significantly decreased, proportional to dose, during the 28 days of dose evaluation in comparison with baseline, as shown in the Figure. No significant mean change in the sUA levels in the combined placebo group was observed. After discontinuation of study drug, sUA levels reverted to pre-treatment levels.
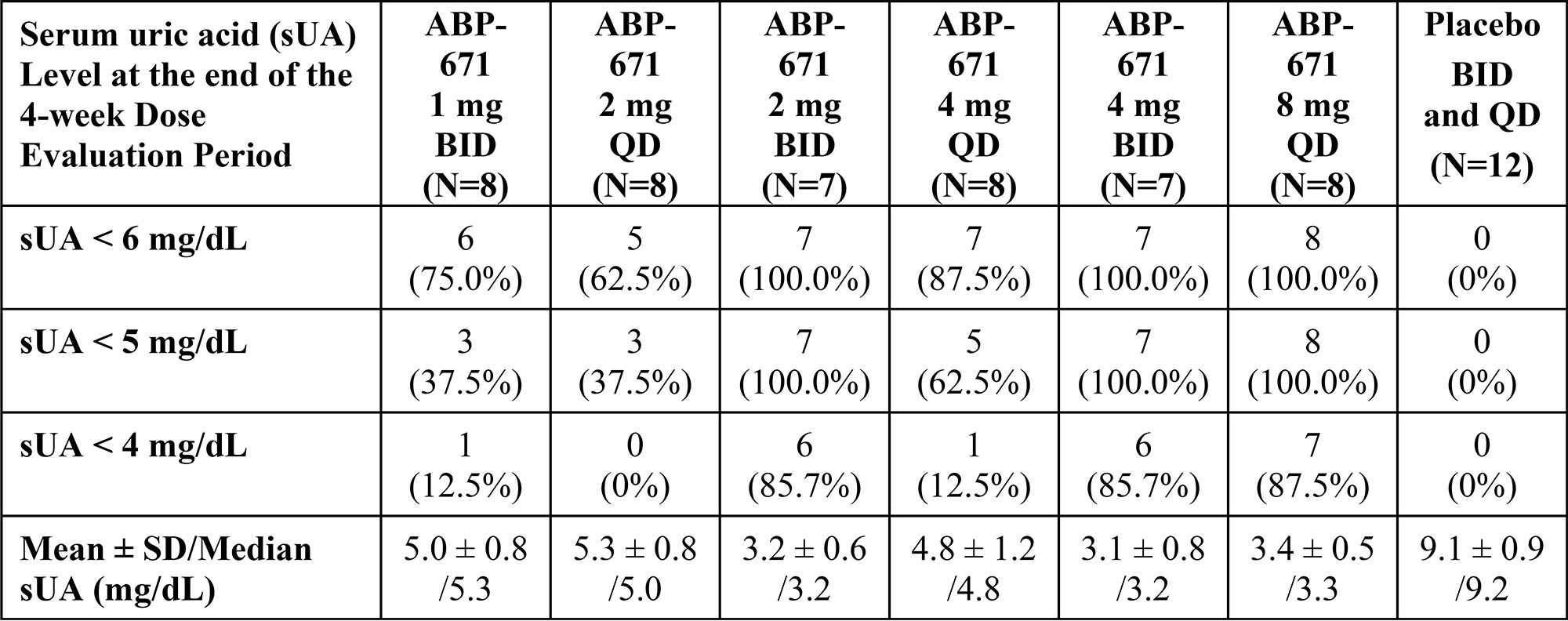
At the end of the 4-week dose evaluation period, the mean sUA levels were 5.0, 5.3, 3.2, 4.8, 3.1, 3.4, and 9.1 mg/dL for the 1 mg BID, 2 mg QD, 2 mg BID, 4 mg QD, 4 mg BID, 8 mg QD and combined placebo cohorts, respectively. sUA levels < 6 mg/dL were achieved by 6 [75%] of 8 participants, 5 [62.5%] of 8 participants, 7 [100%] of 7 participants, 7 [87.5%] of 8 participants, 7 [100%] of 7 participants, and 8 [100%] of 8 participants in the 1 mg BID, 2 mg QD, 2 mg BID, 4 mg QD, 4 mg BID, and 8 mg QD cohorts, respectively. sUA levels < 5 mg/dL were achieved by 3 [37.5%] of 8 participants, 3 [37.5%] of 8 participants, 7 [100%] of 7 participants, 5 [62.5%] of 8 participants, 7 [100%] of 7 participants, and 8 [100%] of 8 participants in the 1 mg BID, 2 mg QD, 2 mg BID, 4 mg QD, 4 mg BID, and 8 mg QD cohorts, respectively. sUA levels < 6 mg/dL were achieved by 0 [0%] of 12 in the combined placebo group, as shown in the Table. ABP-671 was well tolerated at all doses tested. Sixteen (26.7%) participants in the combined ABP-671 dosing groups had gout attacks (flares) on study: 3 (18.8%) of 16 participants in Group 1, 6 (37.5%) of 16 participants in Group 2, 5 (31.3%) of 16 participants in Group 3, and 2 (16.7%) of 12 participants in the combined placebo groups. Three (5.0%) participants experienced nephrolithiasis: 1 (2.1%) in the combined ABP-671 groups and 2 (16.7%) in the combined placebo groups.
Conclusion: ABP-671 induced stable, statistically significant and clinically meaningful decreases in sUA levels at all doses tested compared to placebo and baseline. At the end of the respective dose evaluation periods, an average of 87% of the ABP-671 participants attained target sUA level of < 6 mg/dL, compared to 0% of placebo participants. ABP-671 was well tolerated, without evidence of dose limiting toxicity. These results support further studies of ABP-671 in patients with hyperuricemia or gout.
To cite this abstract in AMA style:
Gurwith M, Smith D, Bird P, Leung J, Bloch M, Kim J, Mohan R, Houston A, Cumming O, Madrid A, Schwertschlag U, Liu J, Wu R, Xu J, Jin A, Shi W. A Double-Blind, Placebo-Controlled, Ascending Dose Phase 2a Study of ABP-671, a Novel, Potent and Selective URAT1 Inhibitor, in Patients with Gout or Hyperuricemia [abstract]. Arthritis Rheumatol. 2022; 74 (suppl 9). https://acrabstracts.org/abstract/a-double-blind-placebo-controlled-ascending-dose-phase-2a-study-of-abp-671-a-novel-potent-and-selective-urat1-inhibitor-in-patients-with-gout-or-hyperuricemia/. Accessed .« Back to ACR Convergence 2022
ACR Meeting Abstracts - https://acrabstracts.org/abstract/a-double-blind-placebo-controlled-ascending-dose-phase-2a-study-of-abp-671-a-novel-potent-and-selective-urat1-inhibitor-in-patients-with-gout-or-hyperuricemia/