Session Information
Session Type: Poster Session B
Session Time: 8:30AM-10:30AM
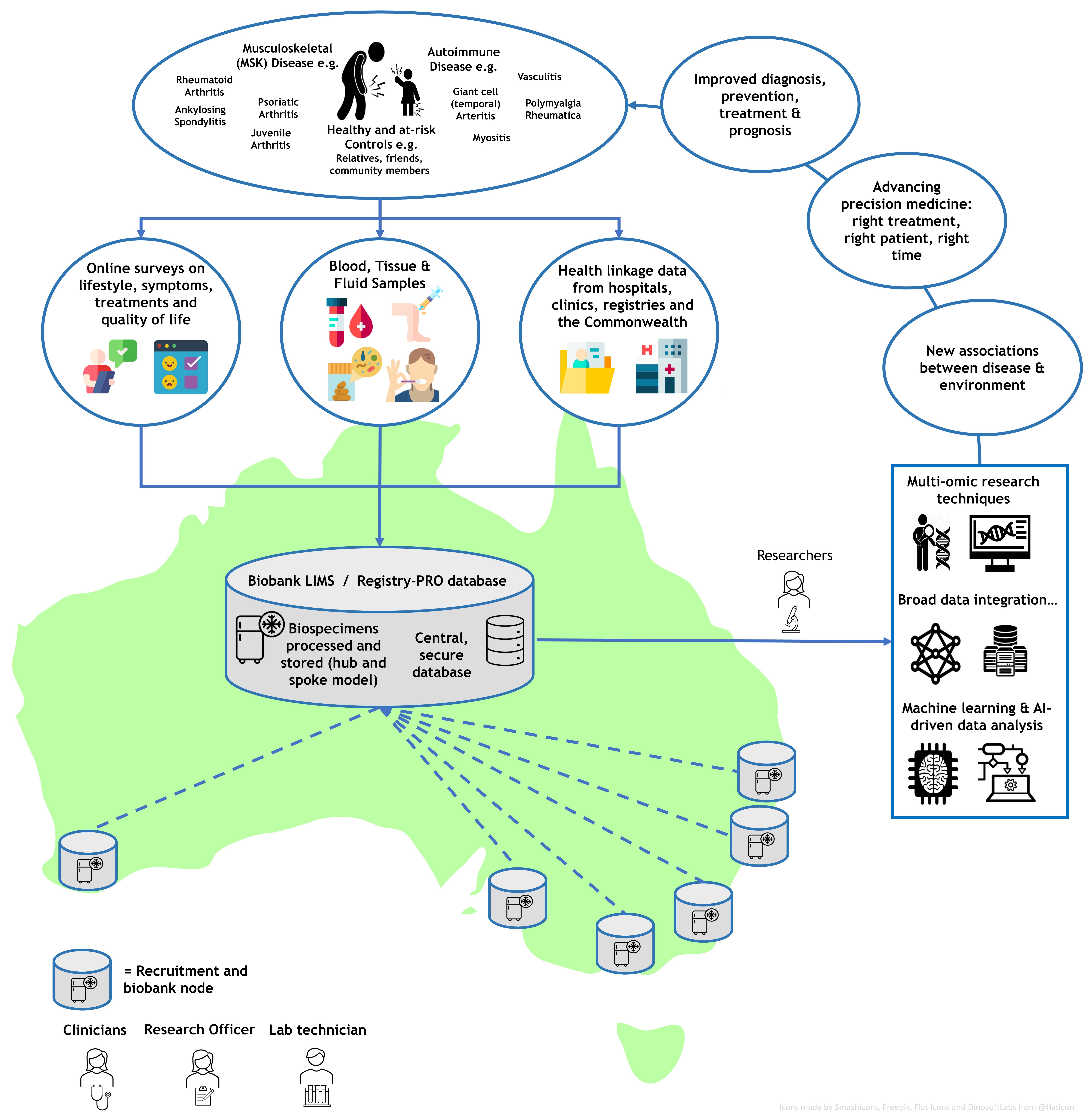
Background/Purpose: As we enter the big data revolution, comprehensive informatics solutions are essential to realising precision medicine for rheumatic and other chronic disease patients, especially for curating high-quality, large-scale and longitudinal biospecimen and linked data collections. In establishing the Australian Arthritis and Autoimmune Biobank Collaborative (A3BC), we sought to develop a low-cost, nation-scale data management system capable of managing our multi-site longitudinal biobank-registry and its complex biobank and data requirements. This included broad life-course data from adults and children across clinical/phenotypic, biological, patient-reported and administrative health data domains, collected to enable holistic multidisciplinary research towards improved outcomes for people living with arthritis and autoimmune conditions (Figure 1).
Methods: We assessed several international commercial and non-profit software platforms using standardised system requirement criteria and follow-up interviews. Vendor compliance scoring was prioritised to meeting our project-critical requirements. Consumer / end-user co-design was integral to refining our system requirements for optimised adoption. Customisation of the selected software solution was performed to optimise field auto-population between participant timepoints and forms, using external modules that do not impact core code. Institutional and independent testing was used to ensure data security.
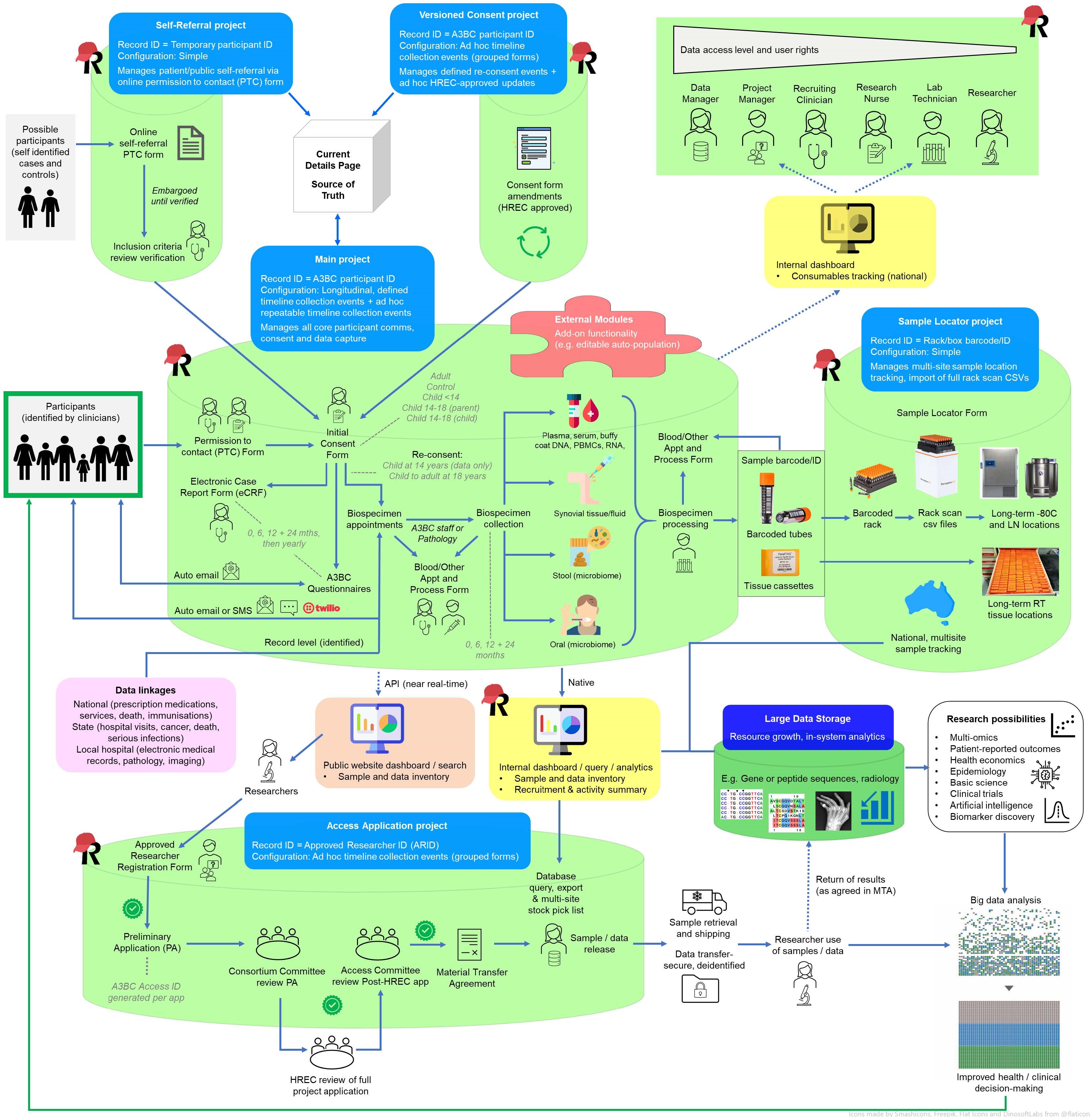
Results: We selected the widely used research web application, Research Electronic Data Capture (REDCap), which is “free” for non-profit REDCap Consortium members. REDCap is highly configurable and customisable to a variety of biobank and registry needs and can be developed/ maintained by end-users with modest IT skill, time and cost. We created a secure, comprehensive participant-centric biobank-registry database that includes best practice data security measures (incl login for multi-site access using academic and government user credentials), permission-to-contact and dynamic itemised e-consent (Figure 2A), a complete chain of custody from consent to biospecimen/data collection to publication, complex longitudinal patient-reported surveys, a fully integrated biobanking workflow (Figure 2B), disease-specific case report forms (Figure 2C), integration of record-level extracted/ linked participant data, significant form auto-population for streamlined data capture, and native dashboards for operational visualisations. The system (Figure 3) has the capacity to enrol participants with a range of diagnoses as well as healthy or at-risk controls (e.g. first degree relatives).
Conclusion: We utilised REDCap to develop an economical, easily-adaptable and sustainable model and recommend it for prospective chronic disease biobanks or biobank-registry projects supporting research into disease prediction, targeted treatments and prevention strategies.
To cite this abstract in AMA style:
Willers C, Lynch T, Chand V, Islam M, Lassere M, Keen H, Kenna T, Lester S, Thomas R, Sinnathurai P, Wechalekar M, Fletcher A, Lightowler D, Mohd S, Ramnoruth N, Ruediger C, Weedon H, March L. A Combined Patient Registry and Biobank Laboratory Information System for Prospective Multisite Chronic Rheumatic Disease Research Using REDCap [abstract]. Arthritis Rheumatol. 2021; 73 (suppl 9). https://acrabstracts.org/abstract/a-combined-patient-registry-and-biobank-laboratory-information-system-for-prospective-multisite-chronic-rheumatic-disease-research-using-redcap/. Accessed .« Back to ACR Convergence 2021
ACR Meeting Abstracts - https://acrabstracts.org/abstract/a-combined-patient-registry-and-biobank-laboratory-information-system-for-prospective-multisite-chronic-rheumatic-disease-research-using-redcap/