Session Information
Date: Sunday, October 26, 2025
Title: (0593–0640) Systemic Lupus Erythematosus – Diagnosis, Manifestations, & Outcomes Poster I
Session Type: Poster Session A
Session Time: 10:30AM-12:30PM
Background/Purpose: Baseline kidney biopsy is the gold standard for diagnosing incident lupus nephritis (LN). However, in certain cases, obtaining a biopsy may not be feasible due to medical, technical, or patient-specific factors, resulting in a clinical diagnosis of LN. We aimed to evaluate differences in outcomes between biopsy-confirmed and clinically diagnosed LN cases to provide guidance in scenarios where a biopsy cannot be obtained.
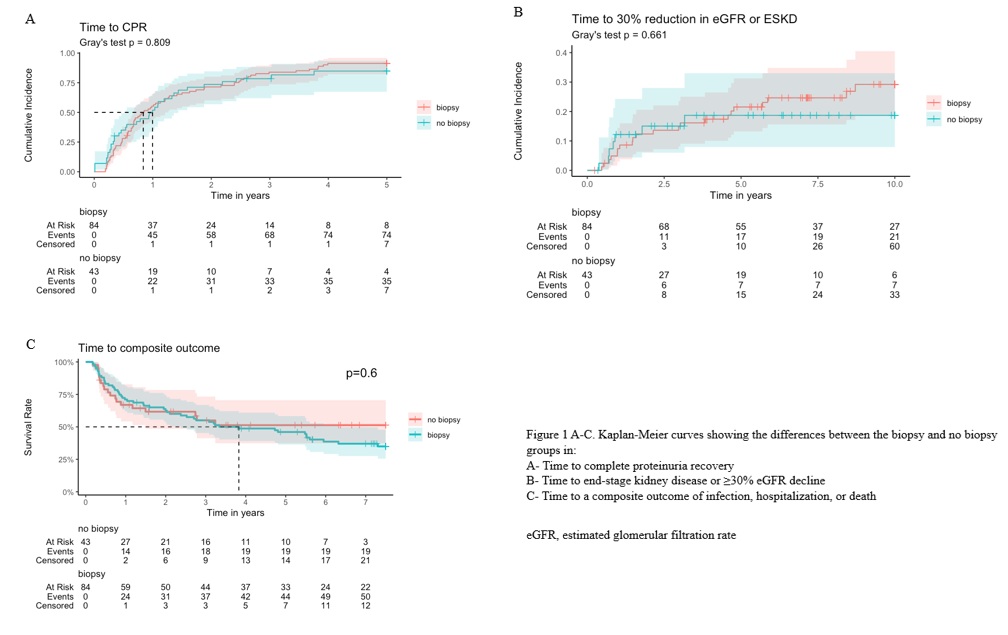
Methods: This retrospective study included inception SLE patients from an observational cohort (2000–2024) who developed incident LN. Patient inclusion began in 2000 to reflect treatment changes associated with the introduction of mycophenolate mofetil. The outcomes assessed were: (1) complete proteinuria recovery (CPR) ( < 0.5 g/g) at one year; (2) progression to end-stage kidney disease (ESKD), defined as eGFR < 15 mL/min/1.73 m², dialysis, or kidney transplantation, or a sustained ≥30% eGFR decline; and (3) a composite adverse outcome, including infection, hospitalization, or death. Time-to-event outcomes were evaluated using Kaplan-Meier (KM) survival curves. Comparisons between biopsy-confirmed and clinically diagnosed LN groups were assessed using Fine-Gray subdistribution hazard models, treating death as a competing risk for the first two outcomes. The models were adjusted for proteinuria as a potential confounder, as well as for imbalanced variables known to clinically impact the outcomes.
Results: 127 patients were included: 84 (66.1%) with biopsy-confirmed LN and 43 (33.9%) with clinically diagnosed LN). Baseline characteristics are summarized in Table 1. Baseline differences were observed in age, proteinuria and albumin levels, SLEDAI-2K, and SLICC/ACR damage index (SDI). Outcomes are summarized in Table 2, and KM curves are provided in Figures 1A-C.< - CPR at 1 Year: Achieved in 58.4% of patients, with no significant differences between groups in rate (p=0.36) and time to event (p=0.81). Adjusted models indicated comparable risk (HR 1.19, 95% CI: 0.76–1.84).< - ESKD or ≥30% Decline in eGFR: Occurred in 22.4% of patients, with no significant differences between groups in rate (p=0.39), time to event (p=0.66), or risk (HR 1.11, 95% CI: 0.46–2.69).< - Composite Outcome: Occurred in 55.1% of the cohort, with no significant differences between groups in rate (p = 0.11), time to event (p = 0.60), or risk (HR 0.99, 95% CI: 0.56–1.74).
Conclusion: Clinically diagnosed incident LN, when treated according to standard-of-care protocols, shows comparable outcomes to biopsy-confirmed LN. While these findings may provide some reassurance, they apply specifically to cases where a baseline biopsy is not feasible and should not be interpreted as a challenge to current diagnostic standards.
To cite this abstract in AMA style:
Kharouf F, Mehta P, Li Q, Gladman D, Touma Z, Whitall Garcia L. A Baseline Kidney Biopsy Is Not Always Feasible in Incident Lupus Nephritis: Insights From an Inception Cohort [abstract]. Arthritis Rheumatol. 2025; 77 (suppl 9). https://acrabstracts.org/abstract/a-baseline-kidney-biopsy-is-not-always-feasible-in-incident-lupus-nephritis-insights-from-an-inception-cohort/. Accessed .« Back to ACR Convergence 2025
ACR Meeting Abstracts - https://acrabstracts.org/abstract/a-baseline-kidney-biopsy-is-not-always-feasible-in-incident-lupus-nephritis-insights-from-an-inception-cohort/

.jpg)
.jpg)