Session Information
Date: Sunday, November 13, 2022
Title: RA – Treatment Poster III
Session Type: Poster Session C
Session Time: 1:00PM-3:00PM
Background/Purpose: SB5, a biosimilar to reference adalimumab (ADL), received marketing authorisation in 2017 (EU) and 2019 (US) based on pre-clinical and clinical phase I and III studies demonstrating bioequivalence and comparable efficacy, safety and immunogenicity to ADL. The real-world study ‘PROPER’ is designed to provide insights into outcomes of the transition from ADL to SB5 outside the randomised, controlled, clinical trial setting.
Methods: Under an umbrella design, 1040 patients with immune-mediated inflammatory disease (rheumatoid arthritis [RA], axial spondyloarthritis [axSpA], psoriatic arthritis [PsA], Crohn’s disease [CD], and ulcerative colitis [UC]) were enrolled at centres in Belgium, Germany, Ireland, Italy, Spain and UK, and followed for 48 weeks post-transition to SB5 after at least 16 weeks’ routine treatment with ADL. This abstract presents results for the RA, axSpA and PsA cohorts.
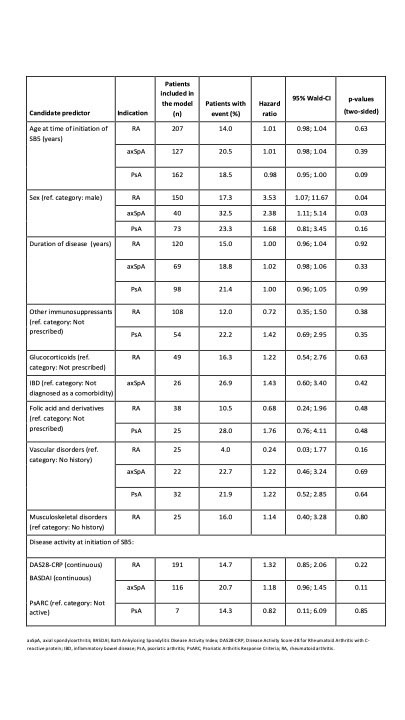
The study aimed to evaluate candidate predictors of persistence on SB5; these variables included gender, medical history, age, duration of disease, disease score and concomitant therapy, all at time of SB5 initiation. Variables reported for at least 15% of each cohort, and having clinical relevance, were selected as candidate predictors, fitted into a univariate Cox regression model, and hazard ratios (HR) calculated for each variable. Persistence on SB5 i.e. time to SB5 discontinuation up to Week 48 post-transition was estimated using the Kaplan-Meier method.
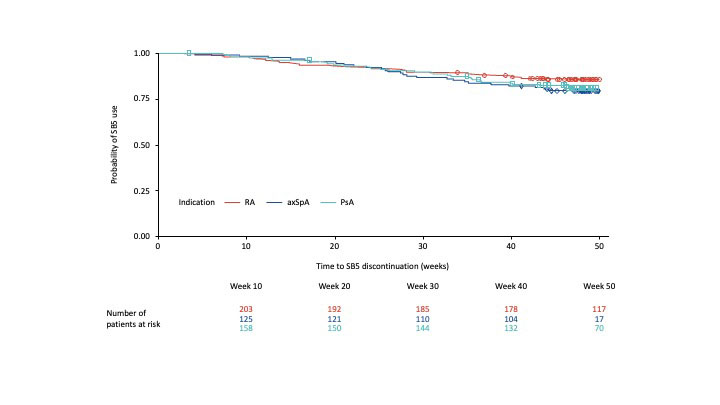
Results: Of the 496 patients included in this analysis, 487 completed 48 weeks of study follow-up; 397 of those (81.5%) remained on SB5 throughout. Probability of persistence at Week 48 was 85.9%, 79.5% and 81.1% for RA, axSpA and PsA, respectively (Figure 1).
Table 1 shows the variables selected as candidate predictors, and the probability of each to predict persistence at Week 48. Being female was the only variable shown to be significantly associated with persistence: HR (95% CI) 3.53 (1.1, 11.7 [p=0.04]) and 2.38 (1.1, 5.1 [p=0.03]) in the RA and axSpA cohorts, respectively. Of the 3 variables with HR ≥1.5, or the reciprocal, the 95% CI crossed 1.0 and the association with persistence was not statistically significant (based on p-value): female gender (in the PsA cohort); history of vascular disorders (in the RA cohort); and receiving folic acid supplement (in the RA and PsA cohorts).
Conclusion: Over three quarters of all three rheumatology indication cohorts remained on SB5 by 48 weeks after transition from reference ADL. Of the candidate predictors of persistence, only female gender was found to have a significant association with increased risk for SB5 discontinuation in study subjects diagnosed with RA or axSpA. A limitation of this finding is the relatively low proportion of subjects discontinuing SB5 prior to Week 48, hence a low incidence of potential candidate predictors within the dataset; p-values should be interpreted with caution. These results suggest that, within the baseline categories explored, no evidence was found to mitigate against transition on grounds of baseline clinical characteristics in terms of anticipated persistence.
To cite this abstract in AMA style:
Müller-Ladner U, Gaffney K, Jadon D, Matucci-Cerinic M, Chamizo Carmona E, Addison J. A 48-week Analysis of a Pan-EU Real-world Study of SB5 Biosimilar Following Transition from Reference Adalimumab in Patients with Rheumatoid Arthritis, Axial Spondyloarthritis or Psoriatic Arthritis [abstract]. Arthritis Rheumatol. 2022; 74 (suppl 9). https://acrabstracts.org/abstract/a-48-week-analysis-of-a-pan-eu-real-world-study-of-sb5-biosimilar-following-transition-from-reference-adalimumab-in-patients-with-rheumatoid-arthritis-axial-spondyloarthritis-or-psoriatic-arthritis/. Accessed .« Back to ACR Convergence 2022
ACR Meeting Abstracts - https://acrabstracts.org/abstract/a-48-week-analysis-of-a-pan-eu-real-world-study-of-sb5-biosimilar-following-transition-from-reference-adalimumab-in-patients-with-rheumatoid-arthritis-axial-spondyloarthritis-or-psoriatic-arthritis/