Session Information
Session Type: Poster Session C
Session Time: 1:00PM-3:00PM
Background/Purpose: More than 10 years ago ACR and EULAR endorsed provisional criteria to define remission in RA, both Boolean and index-based. However, the agreement between these two sets of criteria was moderate, especially if patient global assessment (PGA) was >1 (0-10). Recent studies indicated that a higher threshold for the PGA improved this agreement. We aimed to externally validate a revision of the Boolean remission criteria using a higher PGA threshold and to validate the provisionally endorsed index-based criteria.
Methods: Data from 4 randomized trials of biologic disease modifying antirheumatic drugs (bDMARDs) vs. methotrexate (MTX) or placebo were analyzed. We tested a higher proposed PGA (Visual Analogue Scale; 0-10) threshold of 2 (Boolean 2.0) vs. the original threshold of 1 (Boolean 1.0). We analyzed agreement between the Boolean and index-based criteria (SDAI, CDAI). We further examined how fulfilling each remission definition at 6 months predicted good physical function (HAQ < =0.5) and radiographic non-progression (predictive validity) at one year. A Boolean definition without the PGA (BooleanX) was also assessed.
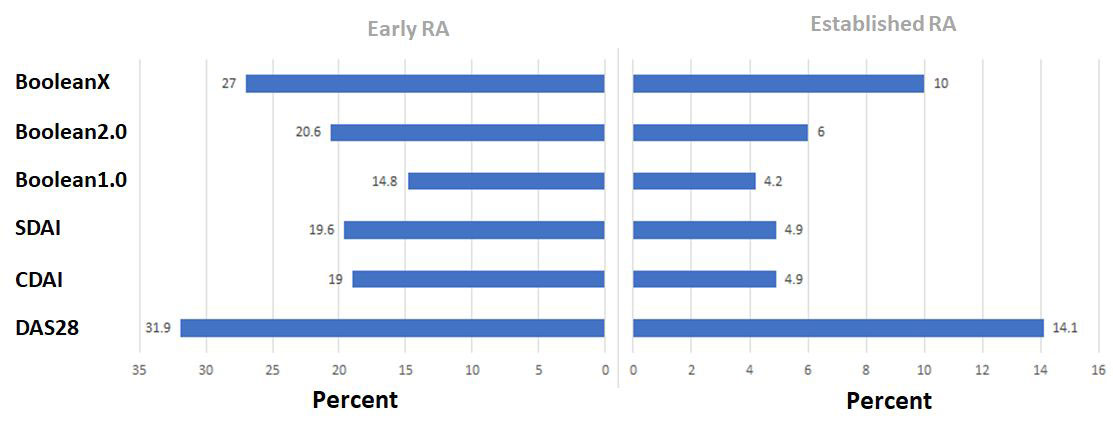
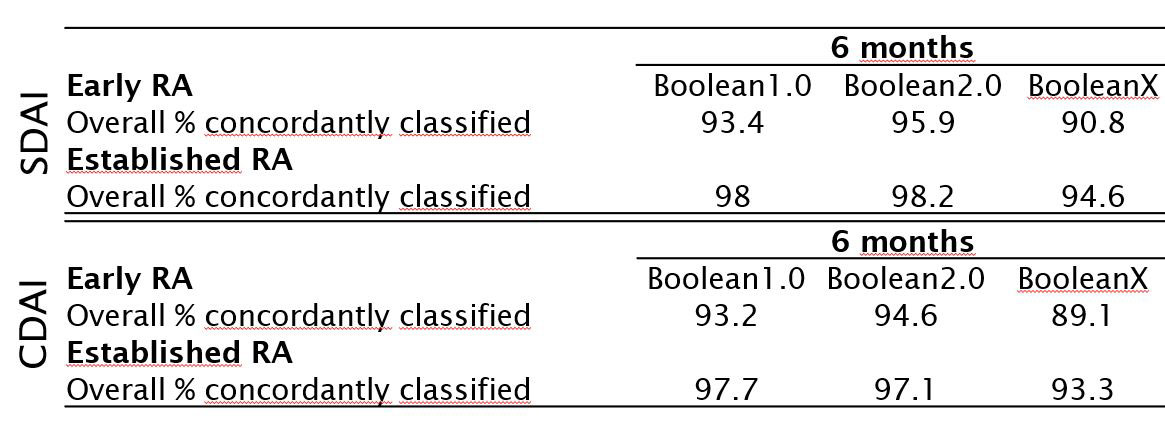
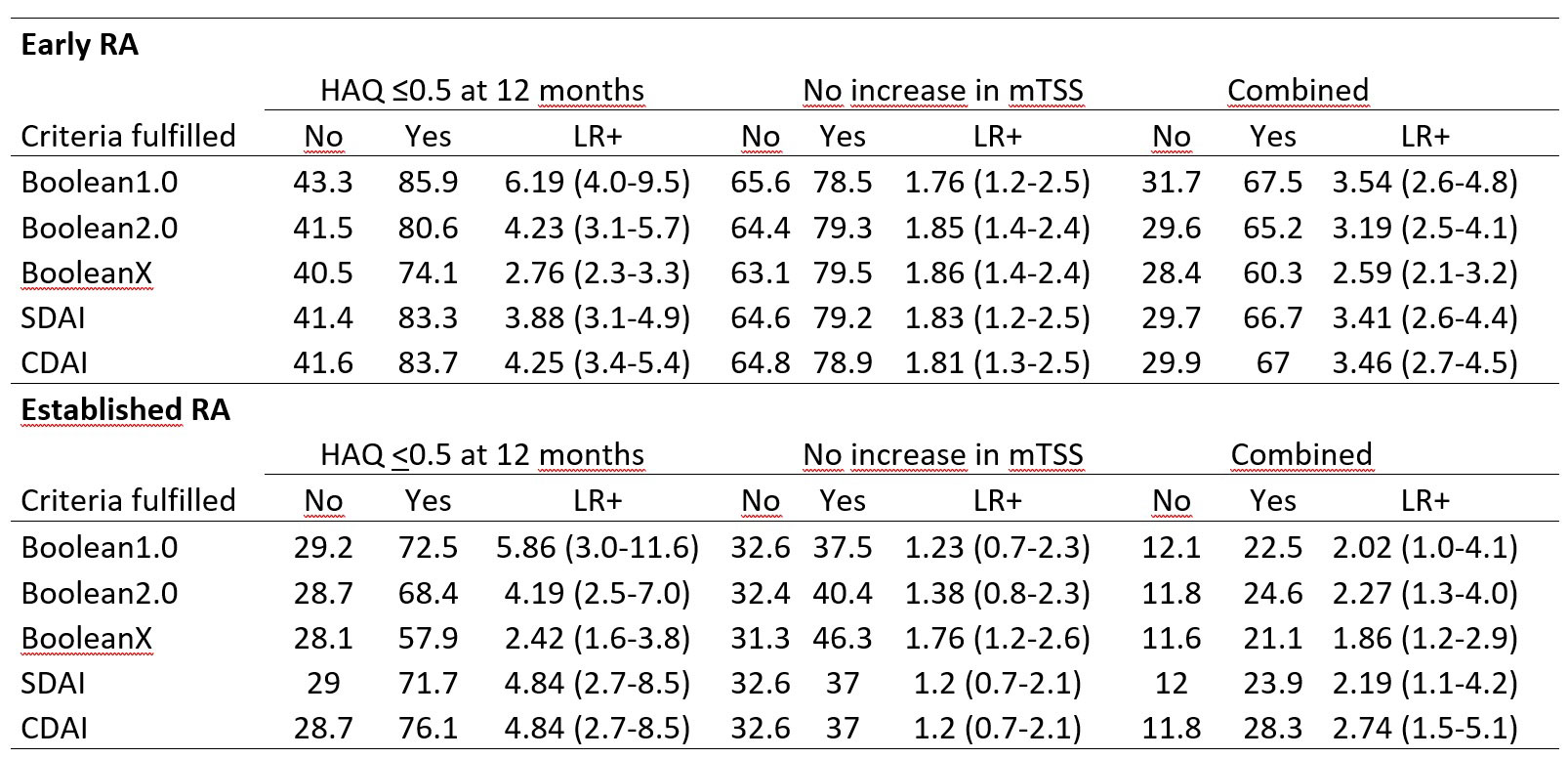
Results: Data from 2048 trial participants, 1101 with early (disease duration ≤2years) and 947 with established RA were included. The proportion of participants in remission at 6 months increased when using Boolean2.0 compared to Boolean1.0 from 14.8% to 20.6% in early RA and 4.2% to 6.0% in established RA (Figure 1). Agreement between Boolean2.0 and the SDAI or CDAI remission criteria was better than for Boolean1.0, particularly in early disease (Table 1). Boolean2.0, SDAI and CDAI remission criteria had similar positive likelihood (LR+) ratios to predict radiographic non-progression and a HAQ of ≤0.5 (Table 2). The proportion of participants achieving both good radiographic and functional outcomes, were similar for all remission definitions, from 57 to 60% (Boolean1.0, 2.0, SDAI and CDAI: 58.6%, 57.3%, 59.2% and 60.4%), except for BooleanX (50.8%).
Conclusion: Using the Boolean2.0 definition increases the agreement with index-based remission criteria and classifies more patients as reaching remission than Boolean 1.0. Boolean2.0, SDAI and CDAI but not definitions of remission without patient global assessment coincide with a good predictive value for both radiographic and functional outcomes.
To cite this abstract in AMA style:
Studenic P, Aletaha D, de Wit M, Stamm T, Alasti F, Lacaille D, Smolen J, Felson D. 2022 Revision of the ACR/EULAR Remission Criteria for Rheumatoid Arthritis [abstract]. Arthritis Rheumatol. 2022; 74 (suppl 9). https://acrabstracts.org/abstract/2022-revision-of-the-acr-eular-remission-criteria-for-rheumatoid-arthritis/. Accessed .« Back to ACR Convergence 2022
ACR Meeting Abstracts - https://acrabstracts.org/abstract/2022-revision-of-the-acr-eular-remission-criteria-for-rheumatoid-arthritis/