Session Information
Session Type: ACR Poster Session C
Session Time: 9:00AM-11:00AM
Background/Purpose: As an extracellular ligand, 14-3-3η potently
and concentration-dependently upregulates the expression of multiple factors
including TNFα, IL-6, and RANKL and its clinical detection is associated
with joint damage progression risk. Several disease modifying agents are
available for the treatment of RA with remission efficacy rates around 30%. Since
RA is driven by multiple factors to varying degrees, within and between
patients along the disease course, personalized medicine that enables the
specific targeting and measurement of disease potentiators, such as
14-3-3η, is highly desirable. This study evaluated the in vivo feasibility
of targeting 14-3-3η with a monoclonal antibody to mitigate the onset and
severity of collagen-induced arthritis (CIA) in mice.
Methods: 27 DBA/1 mice were randomized to four study groups:
non-induced mice (negative control, N=5), 0.5 mg/kg of dexamethasone group
(positive control, N=6), saline injected mice (placebo group, N=10), and the
treatment arm that was administered 10 mg/kg of 14-3-3η mAb (N=6).
Treatments were initiated 2 days prior to immunization with collagen and
administered daily for 6 weeks. A collagen booster was injected on day 18 of
the study for all immunized mice. Arthritis scores were determined daily by an
established and standardized chart evaluating inflammation and swelling of each
paw (0 to 4). All animals were sacrificed 42 days after the beginning of the
treatments. Paws were further analysed by x-ray. Student t-test was performed
to examine differences (onset CIA, maximum, and end scores, and paw scores)
amongst the two groups. Kruskal Wallis test was used to compare group daily
score differences over the course of disease.
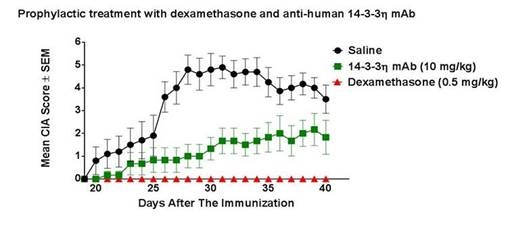
Results: Non-induced and dexamethasone mice did not develop visible
signs of arthritis over the course of disease while 100% of the mice within the
saline arm did. 17% of the mice in the 14-3-3η mAb group did not develop
any signs of arthritis. The CIA score for the 14-3-3η mAb arm had significantly
lower onset scores (0.83 ±0.41 vs 2.7 ±1.57, p=0.0119), maximum scores (2.33
±1.75 vs 5.3 ±1.83, p=0.0052), and end scores (1.83 ±1.84 vs 4.3 ±1.7,
p=0.0197) than the saline treated groups. Figure 1 further
demonstrates that 14-3-3η mAb treated mice
have significantly lower disease over the disease course than
animals in the saline group, p<0.01, with x-ray paw analysis
also demonstrating significance (p=0.0041).
Conclusion: 14-3-3η is a mechanistic joint damage factor
involved in the pathogenesis of RA. A research program to exploit modifying the
14-3-3η pathway is underway to develop improved antibody therapeutics for
delaying the onset and reducing the severity of this disease.
To cite this abstract in AMA style:
Abulrob A, Mercier M, Corluka S, MacKenzie R, Raphael S, Michienzi S, Savill J, Gui Y, Maksymowych W, Marotta A. 14-3-3η As a Novel RA Drug Target: Anti-14-3-3η Monoclonal Antibody Delays the Onset and Mitigates the Severity of Arthritis in CIA Mice [abstract]. Arthritis Rheumatol. 2015; 67 (suppl 10). https://acrabstracts.org/abstract/14-3-3-as-a-novel-ra-drug-target-anti-14-3-3-monoclonal-antibody-delays-the-onset-and-mitigates-the-severity-of-arthritis-in-cia-mice/. Accessed .« Back to 2015 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/14-3-3-as-a-novel-ra-drug-target-anti-14-3-3-monoclonal-antibody-delays-the-onset-and-mitigates-the-severity-of-arthritis-in-cia-mice/