Session Information
Session Type: Abstract Submissions (ACR)
Background/Purpose:
Biomarkers proven to be effective in aSLE patients may not directly apply to cSLE unless validation is done. Existing evidences have shown immunopathogenic roles of C1q and anti-C1q antibody (aC1qA) in SLE both in vitro and in vivo. The latter are strongly associated with the development of lupus nephritis especially in aSLE but evidences of its role in cSLE are rare. We aim to explore the role of aC1qA in our Southeast Asian cSLE cohort in regard to its diagnostic and prognostic properties as our proof-of-concept study.
Methods:
Sixty-eight cSLE patients were recruited and 55 patients with 180 patient-visits with complete clinical data/blood samples were studied. Disease activity indices including SLEDAI-2K, SLAM and BILAG were recorded. Anti-dsDNA, antinucleosome Abs (ANuA, H1-stripped) and aC1qA were measured by ELISA. Patients were evaluated at 1-3 mo intervals depending on their disease severity. Patients were grouped into 3 disease activity groups: no activity (ID), minimal activity (MD) = mild activity with no therapeutic intervention or activity with improvement from previous visit and active disease activity (AD) = new case or flare or persistent activity/refractory to treatment. 72 JIA, 6 JDM, 5 MCTD/UCTD, 11 vasculitides, 5 ANA-positive and 17 other inflammatory conditions composed 116 controls (female 45%, median age (IQR) 13.6 (10.3-16.6) years). Descriptive statistics were used to describe data. Mann-Whitney/Kruskal-Wallis tests were used to compare data and Spearman’s rho for correlation studies.
Results:
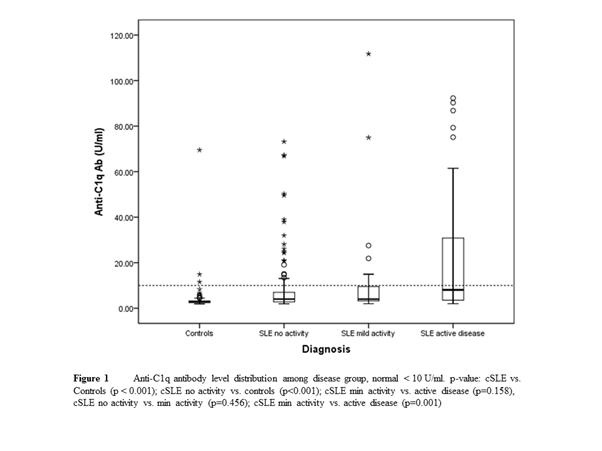
55 cSLE (84% female) with median age of 15.9 (14.4-18.2) yrs and median disease duration of 54.7 (29.4-76.2) mo were included. Majority were Chinese and Malay (38% and 33%). Hematologic disease (98%), arthritis (58%), malar rash (47%) and renal disease (44%) were among most common manifestations. All patients had ANA positivity at onset. Fig 1 shows significant differences in AC1qA levels between controls vs. cSLE and among disease activity groups (p < 0.001). Table 1 reveals strong diagnostic properties of aC1qA. aC1qA was also associated with the presence of nephritis (p=0.034). Correlation analysis showed moderate to good correlations with ESR, C3, C4, anti-dsDNA and ANuA and SLEDAI.
|
Table 1 Anti-C1q antibody as diagnostic biomarker* |
||
|
Properties |
% |
95% confidence interval |
|
Sensitivity |
48.15 |
28.67-68.05 |
|
Specificity |
97.41 |
92.63-99.46 |
|
+ Likelihood ratio |
18.62 |
5.70-60.80 |
|
– Likelihood ratio |
0.53 |
0.37-0.77 |
|
+ Predictive value |
81.25 |
54.35-95.95 |
|
– Predictive value |
88.98 |
82.20-93.84 |
|
Diagnostic odd ratio (DOR) |
35.13 |
|
|
Anti-C1q antibody (aC1q) in cSLE and Controls |
||
|
Parameters |
cSLE (n=180)ᵟ |
Controls (n=116) |
|
aC1q titers (nil < 10 U/ml) |
||
|
No activity (n=106)** |
4.03 (2.78-6.97) |
2.81 (2.46-3.27) |
|
Minimal activity (n=23)** |
4.01 (3.25-9.50) |
|
|
Active disease activity (n=51)** |
8.05 (3.53-30.85) |
|
|
aC1q and |
Correlation coefficients (rho) |
p value |
|
ESR |
0.321 |
<0.001 |
|
CRP |
0.011 |
0.886 |
|
C3 |
-0.573 |
<0.001 |
|
C4 |
-0.486 |
<0.001 |
|
Anti-dsDNA Ab |
0.596 |
<0.001 |
|
Antinucleosome Ab |
0.558 |
<0.001 |
|
SLEDAI |
0.473 |
<0.001 |
|
SLAM |
0.276 |
<0.001 |
|
BILAG |
0.235 |
0.002 |
|
*Controls n=116, cSLE n=27 (20 active disease + 7 newly diagnosed) **Minimal activity = mild activity with no therapeutic intervention or activity with improvement from previous visit Active disease activity = New case or flare or persistent activity/ refractory to treatment †Median (IQR) ᵟpatient-visits |
||
Conclusion:
Our initial findings showed a strong diagnostic and possible, prognostic properties of aC1qA in our cSLE cohort. The presence of aC1qA was associated with lupus nephritis and its levels seem to fluctuate with global, if not only renal disease activity. A longer term and prospective study is needed to validate these initial findings in our region.
Disclosure:
T. Arkachaisri,
None;
J. G. Yeo,
None;
J. H. T. Tan,
None;
S. F. Hoh,
None;
L. Das,
None;
J. Y. Leong,
None.
« Back to 2014 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/does-anti-c1q-antibody-have-diagnostic-and-prognostic-roles-in-childhood-onset-systemic-lupus-erythematosus/