Session Information
Session Type: Abstract Submissions (ACR)
Background/Purpose: Tocilizumab (TCZ) is a recombinant, humanized, monoclonal antibody directed against the cytokine interleukin-6 receptor, and tofacitinib (Tofa) is an oral, synthetic, disease-modifying antirheumatic drug (DMARD) inhibiting Janus kinases (JAKs). Both are licensed for use in combination with other conventional DMARDs or as monotherapy in patients with moderately to severely active rheumatoid arthritis (RA) who have had inadequate responses to ≥1 DMARDs. The purpose of this study was to evaluate the effectiveness of TCZ compared with Tofa in their licensed populations (ie, RA patients with inadequate responses to conventional nonbiologic or biologic DMARDs [cDMARD-IR or bDMARD-IR]) based on publicly available evidence to date.
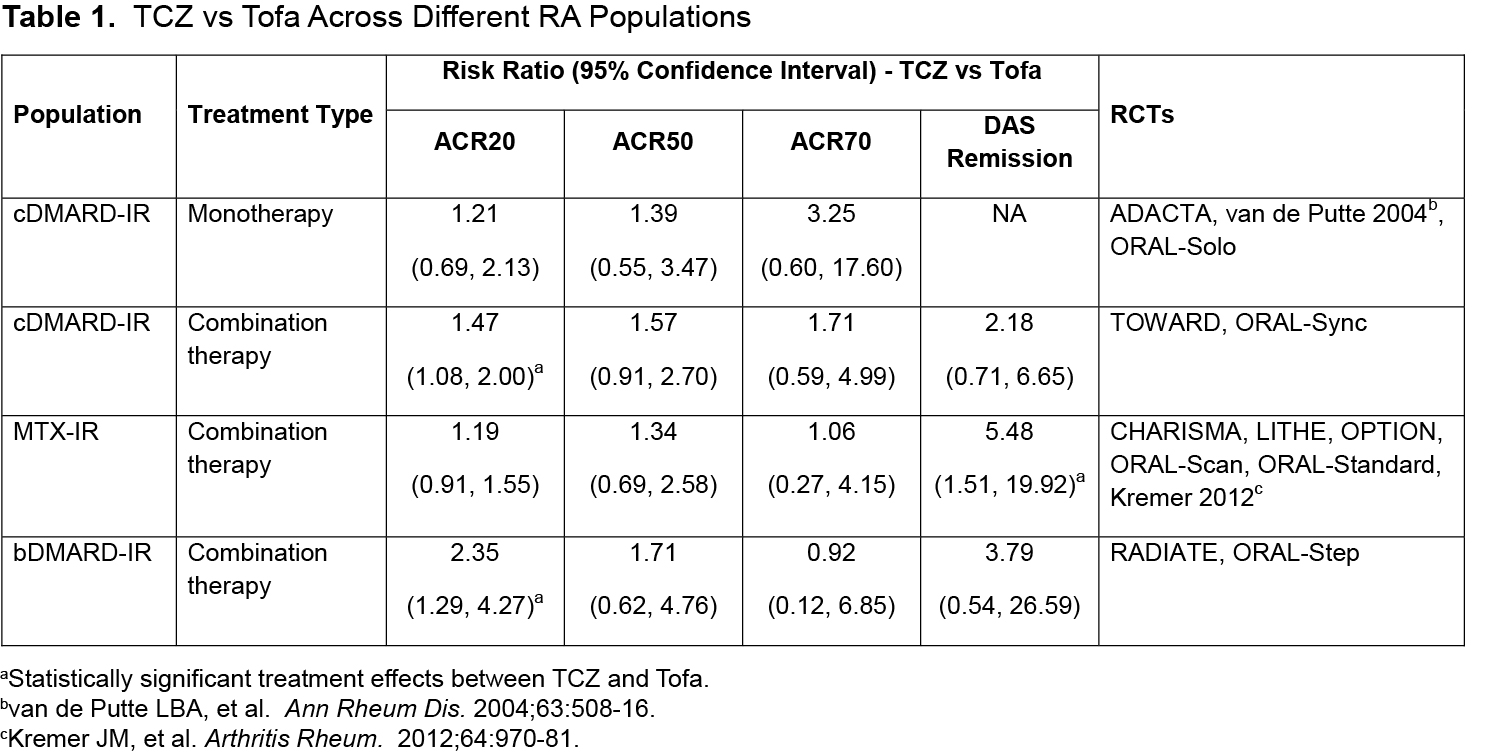
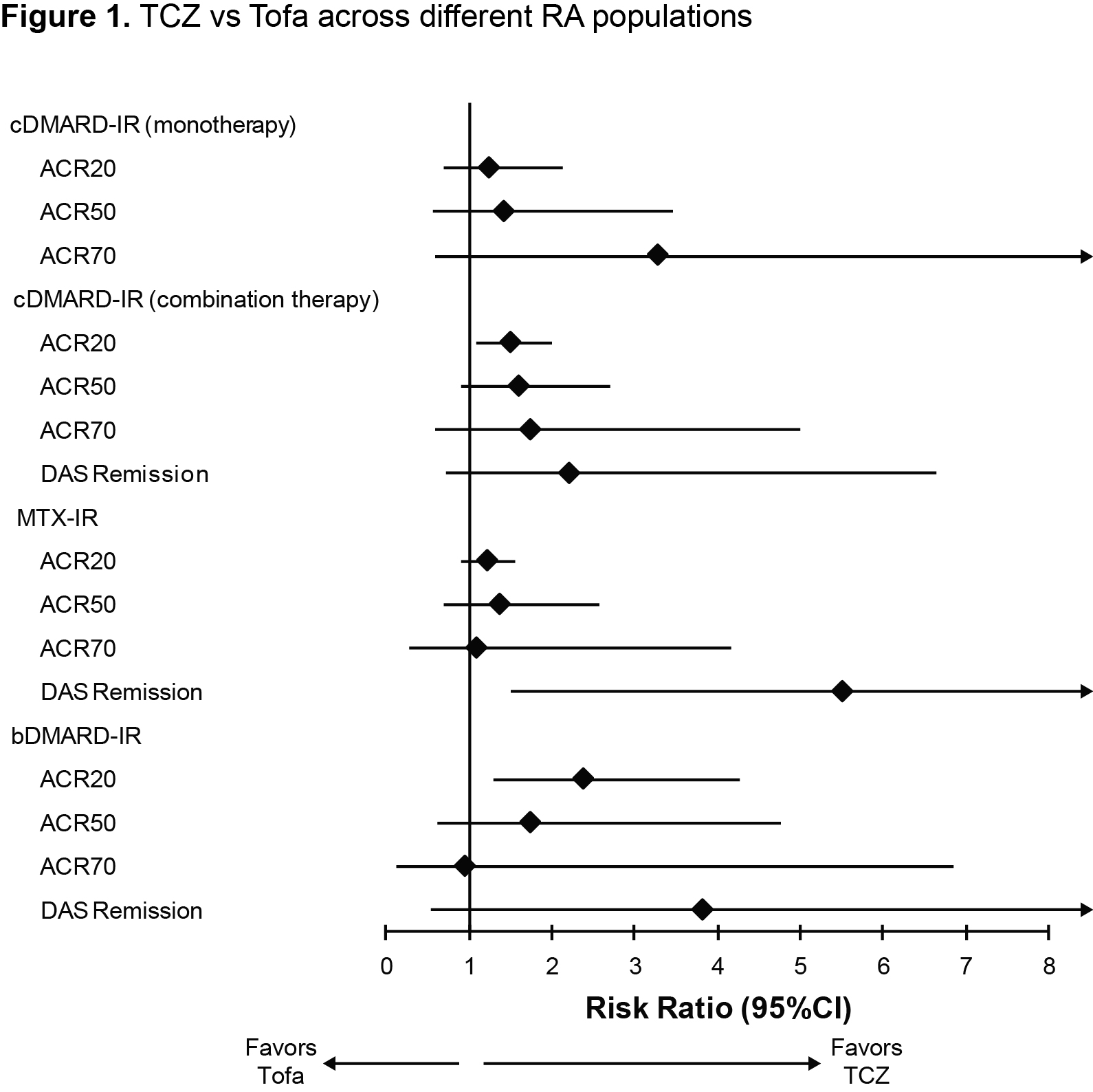
Methods: Efficacy was assessed for each treatment compared with placebo using available randomized controlled trial (RCT) evidence, after which treatments were indirectly compared with each other using the Bucher method.1 Four comparisons were performed, each developed based on similarities between TCZ and Tofa studies in terms of population characteristics and treatment type (monotherapy and combination therapy). Comparative efficacy was assessed on the proportion of patients achieving American College of Rheumatology (ACR) scores of 20, 50, and 70 and Disease Activity Score using 28 joints (DAS28)–defined remission (DAS28 <2.6). Results are presented as risk ratios. Sensitivity analyses were undertaken to test the inclusion/exclusion of studies contributing to statistical heterogeneity.
Results: Across the 4 comparisons, 6 RCTs of TCZ and 6 RCTs of Tofa were included. In both monotherapy and combination therapy in cDMARD-IR and combination therapy in methotrexate (MTX)-IR patients, TCZ was more likely to generate ACR response and DAS remission than Tofa. In bDMARD-IR patients, TCZ in combination with MTX generated more ACR20 and ACR50 responders and DAS remitters than Tofa + MTX. Treatment effects between TCZ and Tofa failed to reach statistical significance in all but 3 instances (Table 1, Figure 1). The trends observed were robust to the sensitivity analyses performed.
Conclusion: Results of the indirect comparisons suggest TCZ and Tofa have broadly similar efficacy across different RA populations. There is a consistent trend (significant in 3 instances) favoring TCZ across all licensed RA populations on the outcome of DAS remission and ACR response.
Reference: 1. Bucher HC et al. J Clin Epidemiol. 1997;50:683-691.
Disclosure:
S. Chang,
F. Hoffmann-La Roche,
5;
L. Sawyer,
F. Hoffmann-La Roche,
5;
F. Dejonckheere,
F. Hoffmann-La Roche,
5.
« Back to 2014 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/indirect-comparison-of-tocilizumab-and-tofacitinib-in-patients-with-rheumatoid-arthritis/