Session Information
Session Type: Abstract Submissions (ACR)
Background/Purpose This study is aimed to investigate the role of umbilical cord derived mesenchymal stem cells (UC-MSCs) on autophagy and apoptosis in T cells from SLE patients, and to explore the underline mechanisms involved in this process.
Methods Peripheral blood mononuclear cells (PBMC) were isolated from SLE patients and healthy donors, and cultured under stimulation with anti-CD3/28 antibodies in the presence or absence of autophagy inhibitor 3-MA (5mM for 6h) or activator rapamycin (50nM for 48h). Autophagy levels and apoptotic rates were measured by flow cytometry with the detection of LC3IIB and Annexin V respectively. To determine the effects of MSCs on T cell autophagy and apoptosis, UC-MSCs were cocultured with T cells at the ratio of 1:10 directly or in transwell system. To observe the changes of pathways upstream of autophagy after MSC treatment, an AMPK activator was added to the cocultures. Meanwhile, mitochondria transmembrane potential (¦¤¦·m), which was closely related to APPK activation, was marked and measured in MSCs and T cells by MitoTracker Deep Red (MDR).
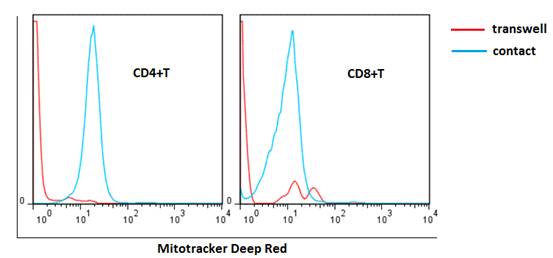
Results T cells from SLE patients had both elevated autophagy level and apoptotic rate compared with those from normal controls, which were further increased after anti-CD3/CD28 stimulation. Apoptotic rate of T cells significantly correlated with autophagy level (r=0.570, p<0.0001 for CD4+T; r=0.508, p=0.0001 for CD8+T). Inhibition of autophagy with 3-MA decreased the apoptotic rate of T cells, whereas activation of autophagy with rapamycin increased the apoptotic rate. UC-MSCs significantly inhibited T cell autophagy (22.5±2.4 vs. 36.4±6.3 for CD4+T; 27.2±1.9 vs. 39.2±5.4 for CD8+T, both p<0.05) and T cell apoptosis (22.2±2.6% vs. 49.1±5.7 % for CD4+T; 23.3±2.4% vs. 53.2±2.3 % for CD8+T, both p<0.05) after cell-to-cell contact coculturing, yet the effect was diminished in transwell system. When AMPK activator added to the cultures, the ability of UC-MSCs to regulated T cell apoptosis was greatly impaired. As shown in Figure 1, mitochondria in UC-MSCs could be transferred to SLE T cells when directly cocultured. Consequently, the elevation of ¦¤¦·m in T cells was downregulated after MSC treatment (242.5±8.4 vs. 315.8±5.5, p=0.003 for CD4+T; 139.8±23.5 vs. 199±35.7, p=0.06 for CD8+T), along with the reduction of AMPK.
Conclusion Autophagy levels are elevated in T cells from SLE patients, leading to aberrant apoptosis. UC-MSCs may inhibit T cell autophagy and apoptosis through mitochondrial transfer.
Figure 1 Transfer of MSC mitochondria to lupus T cells. Mitochondria in MSCs were marked by MitoTracker Deep Red. After cell-to-cell contact coculture, MSC-derived mitochondria were detectable in T cells.
Disclosure:
J. Chen,
None;
X. Feng,
None;
L. Sun,
None.
« Back to 2014 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/uc-mscs-inhibit-t-cell-autophagy-and-apoptosis-in-patients-with-systemic-lupus-erythematosus-through-mitochondrial-transfer/