Session Information
Session Type: Poster Session
Session Time: 10:30AM-12:30PM
Background/Purpose: Systemic lupus erythematosus (SLE) features heterogeneous clinical manifestations. The identification of biomarkers that facilitate initial disease recognition is a cornerstone of optimized clinical management. This study was aimed at summarizing and providing an exhaustive and penetrating exploration of biomarkers associated with organ involvement.
Methods: Embase, PubMed and Web of Science were systematically searched from inception through June 1, 2023. Effect estimates were pooled by the DerSimonian-Laird random-effects model in the presence of substantial heterogeneity (I² >50% or the p-value for Q was < 0·1). Fixed-effects models were not used when heterogeneity criteria were not met. Bivariate models were employed for summarizing diagnostic accuracy parameters when ≥4 datasets were available. Univariate models were used for three or fewer datasets or when bivariate models failed to converge.
Results: A total of 698 studies were included in the analysis and comprised 114,457 lupus patients from 55 countries and territories. The most commonly involved organ and system were renal, neuropsychiatric, hematologic, cardiopulmonary, and cutaneous, corresponding to five distinct lupus phenotypes: lupus nephritis (LN), neuropsychiatric SLE (NPSLE), hematologic involvement, cardiopulmonary involvement, and cutaneous lupus erythematosus (CLE). Thirty-seven biomarkers were significantly associated with the risk of multi-organ involvement. We further analyzed the specific biomarkers for the five phenotypes. It showed that 71 circulating biomarkers were significantly related to an increased risk of LN. Among them, the most frequently reported biomarker was anti-complement 1q (anti-C1q) (odds ratio [OR] 3.76, 95% confidence interval [CI] 2.50–5.64, p < 0.0001). Of the 43 circulating biomarkers correlated with an increased risk of NPSLE, anti-ribosomal P protein (anti-Rib-P) isotypes (anti-Rib-P IgG [OR 1.93, 95% CI 1.10–3.39, p=0.023]) and subtypes (anti-Rib-P0 [OR 2.28, 95% CI 1.20–4.33, p < 0.0001] and anti-neuronal antibodies (OR 6.10, 95% CI 1.65–22.59, p=0.0070) were most frequently reported. The most frequently reported biomarkers for hematologic involvement were platelet-related and coagulation-associated antibodies, as well as non-criteria anti-phospholipid antibodies. High-sensitivity C-reactive protein (hsCRP) levels differed greatly between patients with and without serositis, pericarditis, myocarditis and pleuritis. Anti-nuclear ribonucleoprotein (anti-nRNP) antibodies (OR 3.66, 95% CI 1.94–6.89, p < 0.0001) were the most commonly reported biomarker showing a relationship with a higher risk of pulmonary arterial hypertension. Among the eight specific biomarkers for CLE in SLE patients, anti-SSA60 was significantly associated with increased risk of CLE with the largest number of involved patients (OR 1.30, 95% CI 1.05–1.62, p=0.017).
Conclusion: This large-scale analysis revealed biomarker profiles associated with organ involvement in SLE and provided a clinically actionable non-invasive tool for the risk prediction of organ damage.
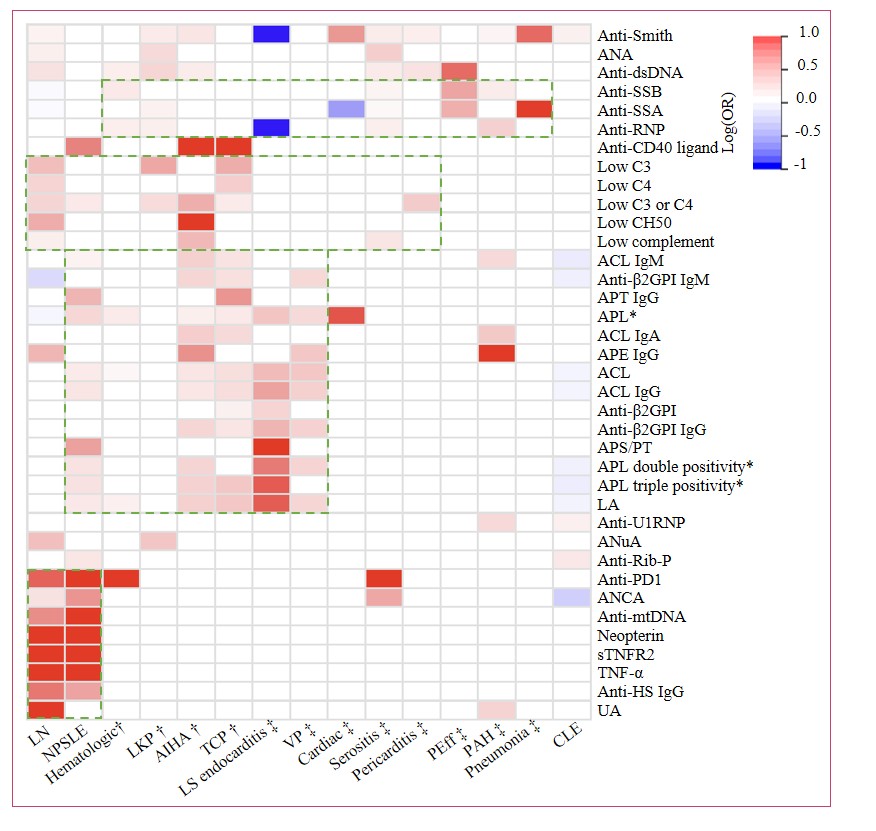 Figure 1: Summary of biomarkers associated with multi-organ involvement in patients with SLE. ACL=anti-cardiolipin antibodies. ANA=anti-nuclear antibodies. Anti-dsDNA=anti-double-stranded DNA antibodies. ANCA=anti-neutrophil cytoplasmic antibodies. Anti-CD40 ligand=anti-CD40 ligand antibodies. Anti-HS=anti-heparan sulfate antibodies. Anti-mtDNA=anti-mitochondrial DNA antibodies. Anti-PD1=anti-programmed cell death protein 1 antibodies. Anti-RNP=anti-ribonucleoprotein antibodies. Anti-Rib-P=anti-ribosomal P protein antibodies. Anti-U1RNP=anti-U1 small nuclear ribonucleoprotein antibodies. Anti-SSA=anti-Sjögren’s syndrome-related antigen A antibodies. Anti-SSB=anti-Sjögren’s syndrome-related antigen B antibodies. Anti-β2GPⅠ=anti-beta-2 glycoprotein I antibodies. Anti-PT=anti-prothrombin antibodies. APE=anti-phosphatidylethanolamine antibodies. APL=anti-phospholipid antibodies. APS/PT=anti-phosphatidylserine/prothrombin antibodies. ANuA=anti-nucleosome antibodies. CLE=cutaneous lupus erythematosus. IgA=immunoglobulin A. IgG=immunoglobulin G. IgM=immunoglobulin M. LA=lupus anticoagulant. Low CH50=low total hemolytic complement activity. LKP=leukopenia. LNflupus nephritis. LS endocarditis=Libman-Sacks endocarditis. NA=not available. NPSLE=neuropsychiatric systemic lupus erythematosus. PAH=pulmonary arterial hypertension. Peff=pericardial effusion. SLE=systemic lupus erythematosus. sTNFR2=soluble tumor necrosis factor receptor 2. TCP=Thrombocytopenia. TNF-α=tumor necrosis factor-alpha. UA=uric acid. VP=valvulopathy. A log(OR) > 0 indicates that biomarker positivity is associated with an increased risk of involvement, while a log(OR) < 0 indicates an association with a decreased risk. *APL include aCL, anti-β2GPI, LA, aPT, aPE, and aPS/PT; among these, aPL double positivity is defined as the concurrent presence of aCL and anti-β2GPI antibodies, while aPL triple positivity is defined as the concurrent presence of aCL, anti-β2GPI, and LA. †Hematologic involvement. ‡Cardiopulmonary involvement.
Figure 1: Summary of biomarkers associated with multi-organ involvement in patients with SLE. ACL=anti-cardiolipin antibodies. ANA=anti-nuclear antibodies. Anti-dsDNA=anti-double-stranded DNA antibodies. ANCA=anti-neutrophil cytoplasmic antibodies. Anti-CD40 ligand=anti-CD40 ligand antibodies. Anti-HS=anti-heparan sulfate antibodies. Anti-mtDNA=anti-mitochondrial DNA antibodies. Anti-PD1=anti-programmed cell death protein 1 antibodies. Anti-RNP=anti-ribonucleoprotein antibodies. Anti-Rib-P=anti-ribosomal P protein antibodies. Anti-U1RNP=anti-U1 small nuclear ribonucleoprotein antibodies. Anti-SSA=anti-Sjögren’s syndrome-related antigen A antibodies. Anti-SSB=anti-Sjögren’s syndrome-related antigen B antibodies. Anti-β2GPⅠ=anti-beta-2 glycoprotein I antibodies. Anti-PT=anti-prothrombin antibodies. APE=anti-phosphatidylethanolamine antibodies. APL=anti-phospholipid antibodies. APS/PT=anti-phosphatidylserine/prothrombin antibodies. ANuA=anti-nucleosome antibodies. CLE=cutaneous lupus erythematosus. IgA=immunoglobulin A. IgG=immunoglobulin G. IgM=immunoglobulin M. LA=lupus anticoagulant. Low CH50=low total hemolytic complement activity. LKP=leukopenia. LNflupus nephritis. LS endocarditis=Libman-Sacks endocarditis. NA=not available. NPSLE=neuropsychiatric systemic lupus erythematosus. PAH=pulmonary arterial hypertension. Peff=pericardial effusion. SLE=systemic lupus erythematosus. sTNFR2=soluble tumor necrosis factor receptor 2. TCP=Thrombocytopenia. TNF-α=tumor necrosis factor-alpha. UA=uric acid. VP=valvulopathy. A log(OR) > 0 indicates that biomarker positivity is associated with an increased risk of involvement, while a log(OR) < 0 indicates an association with a decreased risk. *APL include aCL, anti-β2GPI, LA, aPT, aPE, and aPS/PT; among these, aPL double positivity is defined as the concurrent presence of aCL and anti-β2GPI antibodies, while aPL triple positivity is defined as the concurrent presence of aCL, anti-β2GPI, and LA. †Hematologic involvement. ‡Cardiopulmonary involvement.
.jpg) Figure 2: Effect size of key biomarkers on organ involvement in SLE patients. (A) OR of LN in patients with positive versus negative biomarkers. (B) MD of biomarkers in patients with versus without LN. (C) OR of NPSLE in patients with positive versus negative biomarkers. (D) MD of biomarkers in patients with versus without NPSLE. (E) OR of hematologic involvement in patients with positive versus negative biomarkers. (F) OR of cardiopulmonary involvement in patients with positive versus negative biomarkers. (G) MD of biomarkers in patients with versus without cardiopulmonary involvement. (H) OR of systemic involvement in CLE patients with positive versus negative biomarkers. ALT=alanine aminotransferase. ANA=antinuclear antibodies. Anti-50-kD protein=Anti-50-kD neuronal membrane protein. Anti-A08 C1q=anti-A08 complement component 1q antibodies. Anti-C1q=anti-complement component 1q antibodies. Anti-C1qCLR=anti-C1q collagen-like region antibodies. Anti-C1qGR=anti-C1q globular region antibodies. Anti-CD4 IgM=anti-cluster of differentiation 4 immunoglobulin M antibodies. Anti-cHSP60=anti-chaperonin heat shock protein 60 antibodies. Anti-dsDNA=anti-double-stranded DNA. Anti-ETRA=anti-endothelin receptor type A antibodies. Anti-GABARB=anti-gamma-aminobutyric acid type B receptor subunit. Anti-his=anti-histone antibodies. Anti-Jo-1=anti-histidyl-tRNA synthetase antibodies. Anti-MAP-2=anti-microtubule-associated protein 2 antibodies. Anti-NR=anti-N-methyl-D-aspartate receptor. Anti-nRNP=anti-nuclear ribonucleoprotein antibodies. Anti-PDGFR-α=anti-platelet-derived growth factor receptor alpha antibodies. Anti-Rib-P=anti-ribosomal P protein antibodies. Anti-TPOR=anti-thrombopoietin receptor antibodies. Anti-UCL=anti-ubiquitin carboxyl hydrolase-L1 antibodies. APA=anti-phosphatidic acid antibodies. API=anti-phosphatidylinositol antibodies. APS=anti-phosphatidylserine antibodies. APT=anti-prothrombin antibodies. ATP=anti-thromboplastin antibodies. ATSA=anti-thrombospondin-1 antibodies. BUNfblood urea nitrogen. CXCL13=C-X-C motif chemokine ligand 13. hsCRP=high-sensitivity C-reactive protein. IFNfinterferon. IL=interleukin. LNflupus nephritis. LS endocarditis=Libman-Sacks endocarditis. MD=mean difference. MCP-1=monocyte chemoattractant protein-1. NT-proBNP=N-terminal pro-brain natriuretic peptide. NPSLE=neuropsychiatric SLE. OR=odds ratio. RF=rheumatoid factor. P-C4d=platelet C4d. SLE=systemic lupus erythematosus. SMD=standardized mean difference. TC=total cholesterol. TWEAK=tumor necrosis factor-like weak inducer of apoptosis.
Figure 2: Effect size of key biomarkers on organ involvement in SLE patients. (A) OR of LN in patients with positive versus negative biomarkers. (B) MD of biomarkers in patients with versus without LN. (C) OR of NPSLE in patients with positive versus negative biomarkers. (D) MD of biomarkers in patients with versus without NPSLE. (E) OR of hematologic involvement in patients with positive versus negative biomarkers. (F) OR of cardiopulmonary involvement in patients with positive versus negative biomarkers. (G) MD of biomarkers in patients with versus without cardiopulmonary involvement. (H) OR of systemic involvement in CLE patients with positive versus negative biomarkers. ALT=alanine aminotransferase. ANA=antinuclear antibodies. Anti-50-kD protein=Anti-50-kD neuronal membrane protein. Anti-A08 C1q=anti-A08 complement component 1q antibodies. Anti-C1q=anti-complement component 1q antibodies. Anti-C1qCLR=anti-C1q collagen-like region antibodies. Anti-C1qGR=anti-C1q globular region antibodies. Anti-CD4 IgM=anti-cluster of differentiation 4 immunoglobulin M antibodies. Anti-cHSP60=anti-chaperonin heat shock protein 60 antibodies. Anti-dsDNA=anti-double-stranded DNA. Anti-ETRA=anti-endothelin receptor type A antibodies. Anti-GABARB=anti-gamma-aminobutyric acid type B receptor subunit. Anti-his=anti-histone antibodies. Anti-Jo-1=anti-histidyl-tRNA synthetase antibodies. Anti-MAP-2=anti-microtubule-associated protein 2 antibodies. Anti-NR=anti-N-methyl-D-aspartate receptor. Anti-nRNP=anti-nuclear ribonucleoprotein antibodies. Anti-PDGFR-α=anti-platelet-derived growth factor receptor alpha antibodies. Anti-Rib-P=anti-ribosomal P protein antibodies. Anti-TPOR=anti-thrombopoietin receptor antibodies. Anti-UCL=anti-ubiquitin carboxyl hydrolase-L1 antibodies. APA=anti-phosphatidic acid antibodies. API=anti-phosphatidylinositol antibodies. APS=anti-phosphatidylserine antibodies. APT=anti-prothrombin antibodies. ATP=anti-thromboplastin antibodies. ATSA=anti-thrombospondin-1 antibodies. BUNfblood urea nitrogen. CXCL13=C-X-C motif chemokine ligand 13. hsCRP=high-sensitivity C-reactive protein. IFNfinterferon. IL=interleukin. LNflupus nephritis. LS endocarditis=Libman-Sacks endocarditis. MD=mean difference. MCP-1=monocyte chemoattractant protein-1. NT-proBNP=N-terminal pro-brain natriuretic peptide. NPSLE=neuropsychiatric SLE. OR=odds ratio. RF=rheumatoid factor. P-C4d=platelet C4d. SLE=systemic lupus erythematosus. SMD=standardized mean difference. TC=total cholesterol. TWEAK=tumor necrosis factor-like weak inducer of apoptosis.
.jpg) Figure 3: The most reported biomarkers for organ involvement in SLE. ALT=alanine aminotransferase. ANA=antinuclear antibodies. Anti-50-kD protein=Anti-50-kD neuronal membrane protein. Anti-C1q=anti-complement component 1q antibodies. Anti-dsDNA=anti-double-stranded DNA. Anti-ETRA=anti-endothelin receptor type A antibodies. Anti-GABARB=anti-gamma-aminobutyric acid type B receptor subunit. Anti-his=anti-histone antibodies. Anti-Jo-1=anti-histidyl-tRNA synthetase antibodies. Anti-MAP-2=anti-microtubule-associated protein 2 antibodies. Anti-NR=anti-N-methyl-D-aspartate receptor. Anti-nRNP=anti-nuclear ribonucleoprotein antibodies. Anti-PDGFR-α=anti-platelet-derived growth factor receptor alpha antibodies. Anti-Rib-P=anti-ribosomal P protein antibodies. Anti-SSA=anti-Sjögren’s syndrome-related antigen A antibodies. Anti-TPOR=anti-thrombopoietin receptor antibodies. Anti-UCL-L1=anti-ubiquitin carboxyl hydrolase-L1 antibodies. APA=anti-phosphatidic acid antibodies. API=anti-phosphatidylinositol antibodies. APS=anti-phosphatidylserine antibodies. APT=anti-prothrombin antibodies. ATP=anti-thromboplastin antibodies. ATSA=anti-thrombospondin-1 antibodies. BUNfblood urea nitrogen. CXCL13=C-X-C motif chemokine ligand 13. hsCRP=high-sensitivity C-reactive protein. IL=interleukin. MCP-1=monocyte chemoattractant protein-1. NT-proBNP=N-terminal pro-brain natriuretic peptide. OR=odds ratio. RF=rheumatoid factor. SLE=systemic lupus erythematosus. SMD=standardized mean difference. TC=total cholesterol. TWEAK=tumor necrosis factor-like weak inducer of apoptosis.
Figure 3: The most reported biomarkers for organ involvement in SLE. ALT=alanine aminotransferase. ANA=antinuclear antibodies. Anti-50-kD protein=Anti-50-kD neuronal membrane protein. Anti-C1q=anti-complement component 1q antibodies. Anti-dsDNA=anti-double-stranded DNA. Anti-ETRA=anti-endothelin receptor type A antibodies. Anti-GABARB=anti-gamma-aminobutyric acid type B receptor subunit. Anti-his=anti-histone antibodies. Anti-Jo-1=anti-histidyl-tRNA synthetase antibodies. Anti-MAP-2=anti-microtubule-associated protein 2 antibodies. Anti-NR=anti-N-methyl-D-aspartate receptor. Anti-nRNP=anti-nuclear ribonucleoprotein antibodies. Anti-PDGFR-α=anti-platelet-derived growth factor receptor alpha antibodies. Anti-Rib-P=anti-ribosomal P protein antibodies. Anti-SSA=anti-Sjögren’s syndrome-related antigen A antibodies. Anti-TPOR=anti-thrombopoietin receptor antibodies. Anti-UCL-L1=anti-ubiquitin carboxyl hydrolase-L1 antibodies. APA=anti-phosphatidic acid antibodies. API=anti-phosphatidylinositol antibodies. APS=anti-phosphatidylserine antibodies. APT=anti-prothrombin antibodies. ATP=anti-thromboplastin antibodies. ATSA=anti-thrombospondin-1 antibodies. BUNfblood urea nitrogen. CXCL13=C-X-C motif chemokine ligand 13. hsCRP=high-sensitivity C-reactive protein. IL=interleukin. MCP-1=monocyte chemoattractant protein-1. NT-proBNP=N-terminal pro-brain natriuretic peptide. OR=odds ratio. RF=rheumatoid factor. SLE=systemic lupus erythematosus. SMD=standardized mean difference. TC=total cholesterol. TWEAK=tumor necrosis factor-like weak inducer of apoptosis.
To cite this abstract in AMA style:
Zang S, Yao R, Wang Y, Zhu D, He J, Li Z. Circulating biomarkers for organ involvement in systemic lupus erythematosus: a series of systematic reviews and meta-analyses [abstract]. Arthritis Rheumatol. 2025; 77 (suppl 9). https://acrabstracts.org/abstract/circulating-biomarkers-for-organ-involvement-in-systemic-lupus-erythematosus-a-series-of-systematic-reviews-and-meta-analyses/. Accessed .« Back to ACR Convergence 2025
ACR Meeting Abstracts - https://acrabstracts.org/abstract/circulating-biomarkers-for-organ-involvement-in-systemic-lupus-erythematosus-a-series-of-systematic-reviews-and-meta-analyses/
