Session Information
Date: Wednesday, October 29, 2025
Title: Abstracts: Vasculitis – Non-ANCA-Associated & Related Disorders II (2699–2704)
Session Type: Abstract Session
Session Time: 12:30PM-12:45PM
Background/Purpose: There is a need for better tools to monitor disease activity in giant cell arteritis (GCA). Prior studies demonstrated that vascular enhancement on cranial vessel wall magnetic resonance imaging (vw-MRI) decreases with treatment of GCA, but whether enhancement increases during relapse is not well known. This study examined changes on vw-MRI during relapse of cranial GCA.
Methods: Patients were prospectively enrolled in the study after receiving a diagnosis of GCA by a rheumatologist. All patients met the 2022 American College of Rheumatology/EULAR classification criteria for GCA. Enrolled patients underwent cranial vw-MRIs at enrollment, month 1, month 6, and month 12. Additional imaging was acquired in the setting of suspected relapse, defined as recurrence or worsening of symptoms of GCA felt to require escalation of immunosuppressive therapies. Neuroradiologists masked to disease activity and other clinical data graded vw-MRI enhancement for several cranial arteries and ocular structures using a previously-validated semiquantitative scoring system. Changes in MRI scores were compared with clinically-determined disease activity and acute phase reactants (APRs).
Results: Fourteen patients were included in the study, all of whom underwent a temporal artery biopsy (TAB), of which 12 (86%) had histologic evidence of GCA. All patients were treated with both high-dose glucocorticoids and adjunctive immunosuppressive therapies (10 treated with tocilizumab, 2 with tocilizumab and methotrexate, and 2 with trial-related study drug). Over the course of the study 4 patients experienced a cranial or ocular relapse, 2 patients experienced a relapse with polymyalgia rheumatica (PMR) without cranial symptoms, and 8 patients were in sustained remission. All 4 patients with a cranial or ocular relapse had increased vw-MRI enhancement in at least one cranial structure. Two patients experiencing relapse of PMR had persistent, but not increased enhancement, while 7 of 8 patients in sustained remission had decreased or normal enhancement on follow-up. In 2 patients experiencing relapse, increased vw-MRI enhancement was seen months prior to clinical signs of relapsing disease. Cranial structures that showed increased enhancement at relapse included the occipital artery, optic nerve sheath, and maxillary artery. Levels of APRs remained normal in most patients, likely impacted by use of tocilizumab.
Conclusion: This study serves as proof-of-concept that vw-MRI may be potentially useful to monitor for relapse in GCA. Abnormal vw-MRI enhancement of cranial and orbital structures is responsive to change and correlates with clinical disease activity. Moreover, vw-MRI is more sensitive to changes in disease activity compared to APRs, suggesting that vw-MRI provides additive information to current biomarkers of GCA. A non-invasive method for accurately assessing disease activity is imperative for high-quality longitudinal care of patients with GCA. These findings expand the repertoire of imaging modalities to study for use in assessing disease activity for GCA.
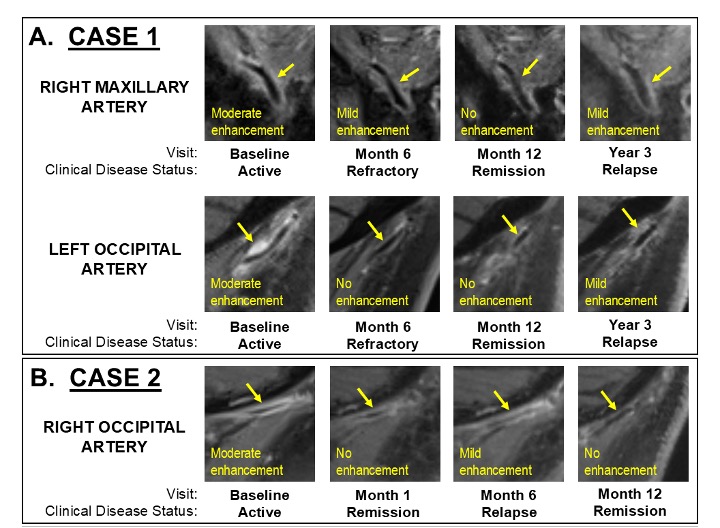 Figure 1. Changes on magnetic resonance imaging prior to and during relapse of giant cell arteritis. Graphs reflect changes in magnetic resonance imaging (MRI) enhancement from most recent visit. MRI enhancement increased during cranial relapse in 4 patients, including 1 patient who experienced increasing enhancement prior to clinical presentation of relapse. MRI enhancement decreased or became normal in 2 patients with a relapse of polymyalgia rheumatica only (non-cranial disease) and in most visits among 8 patients with non-relapsing giant cell arteritis. One patient classified as having non-relapsing giant cell arteritis and who remained in remission during the study period was noted to have increased MRI enhancement at month 12; several months after completion of the study, this patient experienced a relapse, although repeat MRI was not performed at the time of relapse.
Figure 1. Changes on magnetic resonance imaging prior to and during relapse of giant cell arteritis. Graphs reflect changes in magnetic resonance imaging (MRI) enhancement from most recent visit. MRI enhancement increased during cranial relapse in 4 patients, including 1 patient who experienced increasing enhancement prior to clinical presentation of relapse. MRI enhancement decreased or became normal in 2 patients with a relapse of polymyalgia rheumatica only (non-cranial disease) and in most visits among 8 patients with non-relapsing giant cell arteritis. One patient classified as having non-relapsing giant cell arteritis and who remained in remission during the study period was noted to have increased MRI enhancement at month 12; several months after completion of the study, this patient experienced a relapse, although repeat MRI was not performed at the time of relapse.
.jpg) Figure 2. Longitudinal changes in disease activity measures of four patients with relapsing giant cell arteritis (GCA). Figures depict chronological changes in clinical status, medications, combined orbital and cranial vessel wall MRI (vw-MRI), and acute phase reactants (APRs) during enrollment in the 4 patients who experienced cranial or ocular relapse of GCA. (A) In Case 1, although APRs normalized, vw-MRI at month 6 showed persistent abnormal vascular enhancement similar to the patient’s refractory clinical symptoms. Then relapse in year 3 was associated with worsening changes on vw-MRI whereas APRs remained normal, likely due to use of tocilizumab. (B) In Case 2, both vw-MRI and APRs tracked with disease activity in patient enrolled in clinical trial of abatacept. (C) In Case 3, worsening changes on vw-MRI were observed at month 6 prior to developing clinical manifestations of relapse at month 12 whereas APRs were normal throughout likely due to use of tocilizumab. (D) In Case 4, relapse at month 6 was associated with worsening changes on vw-MRI whereas APRs were normal.
Figure 2. Longitudinal changes in disease activity measures of four patients with relapsing giant cell arteritis (GCA). Figures depict chronological changes in clinical status, medications, combined orbital and cranial vessel wall MRI (vw-MRI), and acute phase reactants (APRs) during enrollment in the 4 patients who experienced cranial or ocular relapse of GCA. (A) In Case 1, although APRs normalized, vw-MRI at month 6 showed persistent abnormal vascular enhancement similar to the patient’s refractory clinical symptoms. Then relapse in year 3 was associated with worsening changes on vw-MRI whereas APRs remained normal, likely due to use of tocilizumab. (B) In Case 2, both vw-MRI and APRs tracked with disease activity in patient enrolled in clinical trial of abatacept. (C) In Case 3, worsening changes on vw-MRI were observed at month 6 prior to developing clinical manifestations of relapse at month 12 whereas APRs were normal throughout likely due to use of tocilizumab. (D) In Case 4, relapse at month 6 was associated with worsening changes on vw-MRI whereas APRs were normal.
.jpg) Figure 3. Example images of dynamic changes in vessel wall enhancement on magnetic resonance imaging in two cases of relapsing cranial giant cell arteritis. All magnetic resonance images are sagittal post-contrast T1-weighted black-blood sequences with fat saturation. Visit and clinical disease status are listed below each image. (A) Case 1: right maxillary and left occipital arteries (arrows) at each visit. (B) Case 2: right occipital artery (arrow) at each visit.
Figure 3. Example images of dynamic changes in vessel wall enhancement on magnetic resonance imaging in two cases of relapsing cranial giant cell arteritis. All magnetic resonance images are sagittal post-contrast T1-weighted black-blood sequences with fat saturation. Visit and clinical disease status are listed below each image. (A) Case 1: right maxillary and left occipital arteries (arrows) at each visit. (B) Case 2: right occipital artery (arrow) at each visit.
To cite this abstract in AMA style:
Zeng R, Rebello R, Guggenberger K, Baker J, Banerjee S, Kurtz R, Amudala N, Merkel P, Rhee R. Longitudinal Changes on Cranial Magnetic Resonance Imaging in Relapsing Giant Cell Arteritis [abstract]. Arthritis Rheumatol. 2025; 77 (suppl 9). https://acrabstracts.org/abstract/longitudinal-changes-on-cranial-magnetic-resonance-imaging-in-relapsing-giant-cell-arteritis/. Accessed .« Back to ACR Convergence 2025
ACR Meeting Abstracts - https://acrabstracts.org/abstract/longitudinal-changes-on-cranial-magnetic-resonance-imaging-in-relapsing-giant-cell-arteritis/
