Session Information
Session Type: Abstract Session
Session Time: 12:00PM-12:15PM
Background/Purpose: Guidelines recommend the use of composite scores to evaluate disease activity in RA and inform a treat-to-target approach. It is recognised that patient-reported components of these scores are influenced by factors unrelated to inflammation such as other chronic pain conditions. We assessed whether those with a higher genetic predisposition to pain had higher disease activity scores and which components of disease activity were most affected.
Methods: Participants from the Veterans Affairs RA registry, a longitudinal cohort of veterans with RA, were genotyped using the Infinium Global Screening Array-24 v2.0; imputation was performed using the TOPMed server. We applied two existing polygenic risk scores (PGS) including: 1) pain intensity (PI-PGS) and 2) chronic back pain (CBP-PGS). Scores were normalized and standardized within participants of similar genetic backgrounds. Disease activity components were recorded by treating providers during routine clinical encounters and extracted from the electronic health record. We assessed common disease activity scores including the DAS28, Clinical Disease Activity Index (CDAI), and Routine Assessment of Patient Index Data 3 (RAPID3), as well as modified disease activity scores (M-DAS28, M-SDAI) that replace patient global assessment with provider global assessment and removes tender joint count (TJC). The effect of PI-PGS and CBP-PGS on disease activity scores and their individual components was evaluated with generalized estimating equations to use all available longitudinal data, adjusted for sex, age, and five principal components of population structure and clustered by participant with an exchangeable correlation structure. We evaluated the effect of the genetic risk as a continuous measure and by quartile.
Results: A 1 standard deviation (SD) higher PI-PGS and CBP-PGS were each associated with significantly higher DAS28, CDAI, SDAI, and RAPID3. The strength of the association was more modest for the M-DAS28 and M-SDAI (Fig. 1). A 1 SD increase in each PGS was associated with significantly higher pain score, TJC, patient global scores and HAQ (Table 1). There was no association between a 1SD increase in either PGS and SJC or CRP. A dose-dependent effect of PI-PGS on disease activity scores was observed; there were significant differences between 1st and 4th quartile of PI-PGS for all disease activity scores (Fig 2). The highest quartile had a 0.29 higher average DAS28 compared to the lowest quartile. This difference represents 24% of the minimal clinically important difference described for the DAS28.
Conclusion: Higher genetic predisposition to pain is associated with higher disease activity scores in a dose dependent manner with an effect size that may have clinical relevance. The consistency of the findings using a risk score developed for back pain suggests an association through mechanisms unrelated to RA inflammation. Our study suggests commonly used disease activity scores, especially components related to patient global and TJC, are biased in the setting of chronic pain. Clinicians should interpret disease activity in the context of the presence of other chronic pain conditions and consider using M-DAS28 and M-SDAI in those with chronic pain.
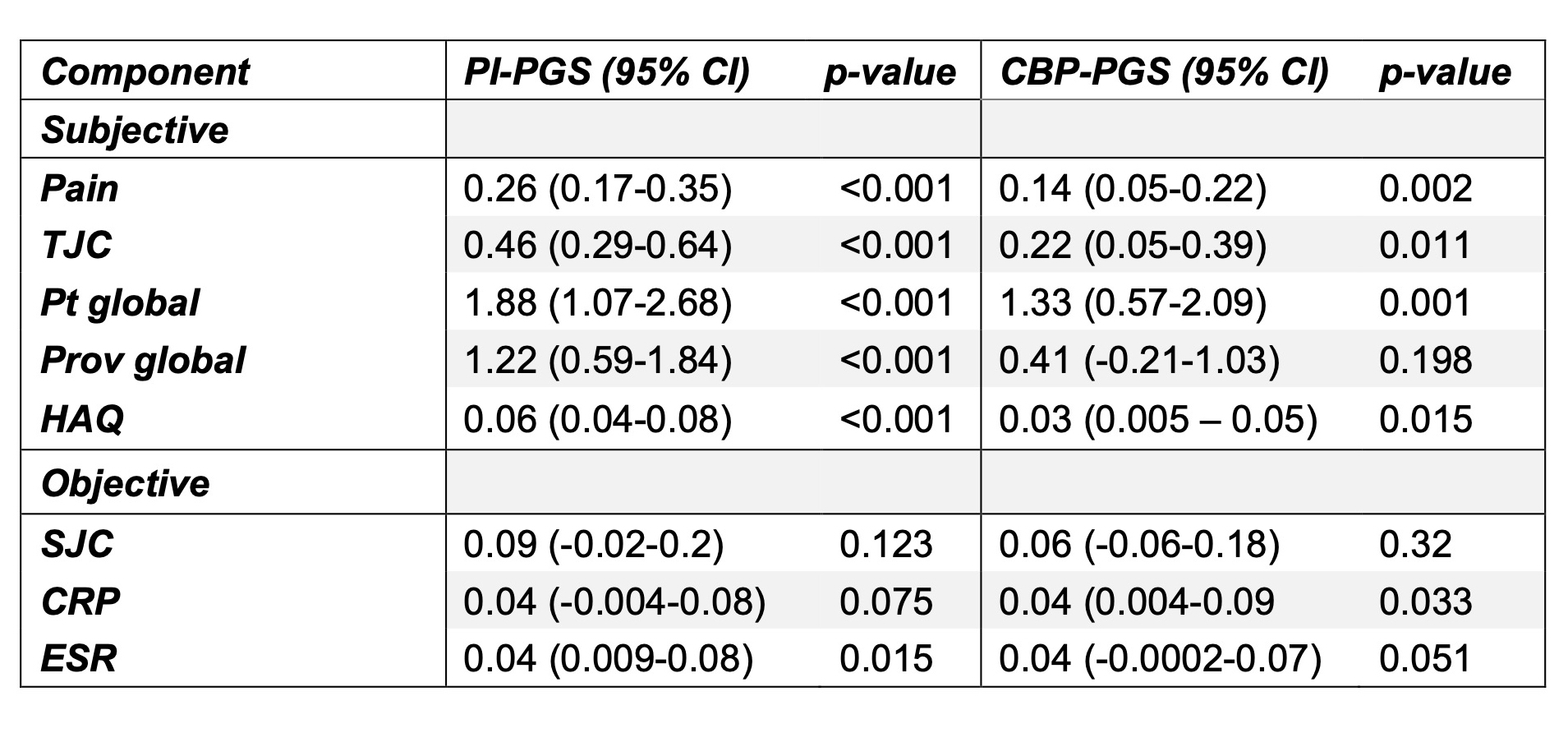 Figure 1: Standardized effect of the pain intensity polygenic score on disease activity scores within VARA. Table and forest plot demonstrating standardised effect size of 1SD increase in PI-PGS on disease activity scores. Similar standardized effects are observed on the traditional disease activity scores; the PI-PGS had a lower standardized effect in the scores modified to remove some patient-reported aspects. Absolute effect sizes are included on the scale of the individual disease activity score.
Figure 1: Standardized effect of the pain intensity polygenic score on disease activity scores within VARA. Table and forest plot demonstrating standardised effect size of 1SD increase in PI-PGS on disease activity scores. Similar standardized effects are observed on the traditional disease activity scores; the PI-PGS had a lower standardized effect in the scores modified to remove some patient-reported aspects. Absolute effect sizes are included on the scale of the individual disease activity score.
Abbreviations
DAS28 – disease activity score-28
CDAI – clinical disease activity index
SDAI – simplified disease activity index
HAQ – health activity questionnaire
RAPID3 – routine assessment of patient index data 3
M-SDAI – modified simplified disease activity index
M-DAS28 – modified disease activity score-28
VARA – Veterans Affairs Rheumatoid Arthritis Registry
PI-PGS – pain intensity polygenic score
.jpg) Table 1: Absolute effect of pain intensity and chronic back pain polygenic scores on components of disease activity scores grouped by patient-reported and objective aspects of disease activity. The PGS demonstrate statistically significant effects on patient-reported aspects of disease activity. The effect of 1 standard deviation change in the PGS on components of disease activity scores was determined through generalized linear models using all available longitudinal data, adjusting for age, sex, and five principal components of population structure.
Table 1: Absolute effect of pain intensity and chronic back pain polygenic scores on components of disease activity scores grouped by patient-reported and objective aspects of disease activity. The PGS demonstrate statistically significant effects on patient-reported aspects of disease activity. The effect of 1 standard deviation change in the PGS on components of disease activity scores was determined through generalized linear models using all available longitudinal data, adjusting for age, sex, and five principal components of population structure.
Abbreviations:
PI-PGS – pain intensity polygenic score
CBP-PGS – chronic back pain polygenic score
TJC – tender joint count (0-28)
Pt global – patient global assessment (0-100)
Prov global – provider global assessment (0-100)
SJC – swollen joint count (0-28)
CRP – C-reactive protein, log-transformed
ESR – erythrocyte sedimentation rate, log-transformed
.jpg) Figure 2. Absolute effect of quartile of pain-intensity polygenic score on DAS28 and M-DAS28. A dose effect is observed from increasing quartile of PI-PGS as compared to 1st quartile on DAS28 and to a more modest extent on M-DAS28. Absolute effect sizes are shown.
Figure 2. Absolute effect of quartile of pain-intensity polygenic score on DAS28 and M-DAS28. A dose effect is observed from increasing quartile of PI-PGS as compared to 1st quartile on DAS28 and to a more modest extent on M-DAS28. Absolute effect sizes are shown.
Abbreviations:
DAS28 – disease activity score-28
M-DAS28 – modified disease activity score-28
To cite this abstract in AMA style:
Barry S, McMenamin K, Wheeler A, England B, Cannon G, Sauer B, Kunkel G, Wysham K, Wallace B, Reimold A, Kerr G, Smith I, Richards J, Lee I, Xiao R, Toikumo S, Kranzler H, Kember R, Damrauer S, Levin M, George M, Mikuls T, Baker J, Riley T. Association of Genetic Risk for Pain Intensity with Longitudinal Disease Activity in Rheumatoid Arthritis [abstract]. Arthritis Rheumatol. 2025; 77 (suppl 9). https://acrabstracts.org/abstract/association-of-genetic-risk-for-pain-intensity-with-longitudinal-disease-activity-in-rheumatoid-arthritis/. Accessed .« Back to ACR Convergence 2025
ACR Meeting Abstracts - https://acrabstracts.org/abstract/association-of-genetic-risk-for-pain-intensity-with-longitudinal-disease-activity-in-rheumatoid-arthritis/
