Session Information
Session Type: Abstract Session
Session Time: 4:15PM-4:30PM
Background/Purpose: Increased pain and decreased function are hallmarks of knee OA progression. Lorecivivint (LOR), an intra-articular CLK/DYRK inhibitor thought to modulate inflammatory and Wnt pathways, appeared safe, improved pain and function outcomes and maintained radiographic medial joint space width (JSW) compared with placebo (PBO) in clinical trials to date. A Phase 3 trial, OA-07 (NCT04520607), evaluated LOR’s safety and assessed WOMAC Pain and Function and JSW outcomes. A post-hoc analysis assessed whether LOR could delay pain and function worsening, proposed predictors of future knee replacement surgery1.
Methods: Patients who had completed the year-long, single-injection LOR trial Study OA-11 (NCT03928184), were invited to enroll into Study OA-07. At OA-07 baseline, patients received the same randomized study injection originally received in Study OA-11 and patients and investigators remained blind to treatment allocation. At the end of OA-07’s first year, all patients were treated with open-label LOR. In this post-hoc analysis, worsening in WOMAC Pain (WOMAC Pain [0-100] increase by 10+ points), worsening in WOMAC Function ( > 9 points), and worsening in coincident WOMAC Pain and Function were explored using multivariable Logistic and Cox regression to assess differences between LOR and PBO in the first year of OA-07 in the Full Analysis Set. Progression in PBO patients during the first year was analyzed compared to their second year after cross-over to LOR, so that each patient served as their own control. The PBO-LOR crossover analysis was analyzed using repeated measures multivariable logistic and Cox regression in the Per-Protocol Analysis Set for complete Year 1 and Year 2 assessments.
Results: 276 patients were enrolled (LOR Nf138, PBO Nf138, 62.7% female, mean age 61.0 ± 8.2 years, mean BMI 31.8 ± 4.9 kg/m2, 45.3% Kellgren-Lawrence Grade 3, average medial JSW 2.63 ± 0.69 mm). LOR appeared safe and well-tolerated with no adverse event rate differences compared to PBO.Significant reduction in the progression of WOMAC Pain (LOR n=38 [29.2%], PBO n=57 [43.2%], OR=1.86, 95% CI [1.12, 2.12], P=0.018), progression of WOMAC Function (LOR n=35 [26.9%], PBO n=52 [39.4%], OR=1.82, 95% CI [1.07, 3.08], P=0.026), and progression in composite Pain and Function (n=28 [21.5%]) PBO (n=45 [34.1%]) (OR=1.90, 95% CI [1.09, 3.31], P=0.023), adjusting for age and KL Grade, were seen with LOR compared to PBO. Time to progression was also significantly reduced in LOR compared to PBO for WOMAC Pain (Figure 1), WOMAC Function (Figure 2), the combined Pain and Function progression (Figure 3). LOR also reduced progression in WOMAC Pain (OR=1.73, 95% CI (0.88, 3.04), P=0.054) and WOMAC Function (OR=2.12, 95% CI (1.16, 3.87), P=0.015) in PBO patients crossed over to LOR.
Conclusion: LOR was shown to be safe and efficacious in Study OA-07. Significant reduction in pain and function progression was seen for LOR compared to PBO, with LOR delaying time to progression. PBO patients who crossed to LOR treatment also demonstrated reduced progression. LOR continues to show promise as a safe, disease-modifying knee OA treatment.References: 1. Kim Y et al. Arthritis Care Res 2022;74(7):1154-1162
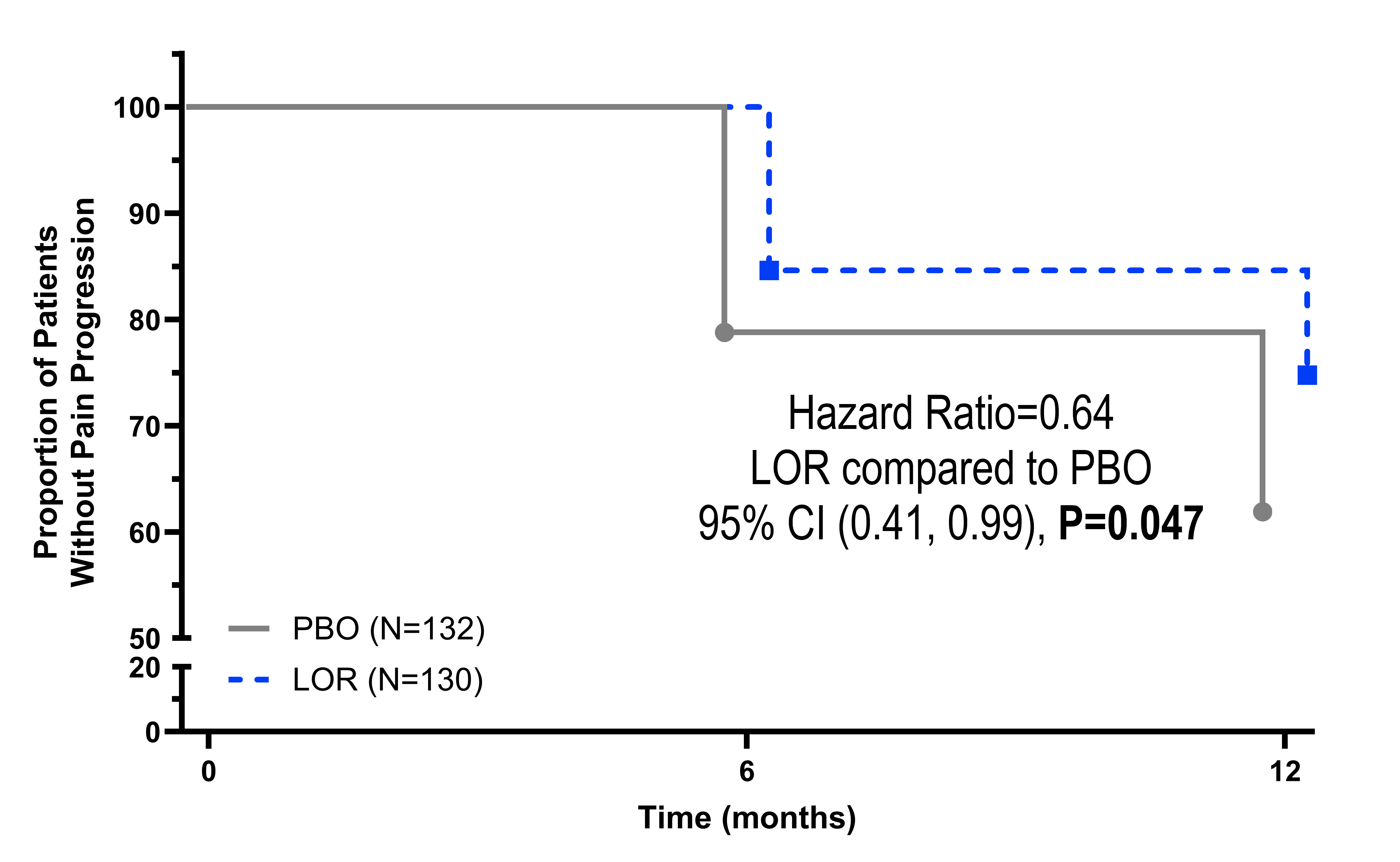 Figure 1. Analysis of LOR compared to PBO in Time to Progression in WOMAC Pain [0-100]
Figure 1. Analysis of LOR compared to PBO in Time to Progression in WOMAC Pain [0-100]
.jpg) Figure 2. Analysis of LOR compared to PBO in Time to Progression in WOMAC Function [0-100]
Figure 2. Analysis of LOR compared to PBO in Time to Progression in WOMAC Function [0-100]
.jpg) Figure 3. Analysis of LOR compared to PBO in Time to Coincident Progression of WOMAC Pain and Function
Figure 3. Analysis of LOR compared to PBO in Time to Coincident Progression of WOMAC Pain and Function
To cite this abstract in AMA style:
Yazici Y, Swearingen C. Lorecivivint Delayed Time to Pain and Function Worsening Compared to Placebo: Evaluation of Knee OA Symptom Progression Outcomes in a Phase 3 Trial (OA-07) [abstract]. Arthritis Rheumatol. 2025; 77 (suppl 9). https://acrabstracts.org/abstract/lorecivivint-delayed-time-to-pain-and-function-worsening-compared-to-placebo-evaluation-of-knee-oa-symptom-progression-outcomes-in-a-phase-3-trial-oa-07/. Accessed .« Back to ACR Convergence 2025
ACR Meeting Abstracts - https://acrabstracts.org/abstract/lorecivivint-delayed-time-to-pain-and-function-worsening-compared-to-placebo-evaluation-of-knee-oa-symptom-progression-outcomes-in-a-phase-3-trial-oa-07/
