Session Information
Date: Tuesday, October 28, 2025
Title: Abstracts: Measures & Measurement of Healthcare Quality (2615–2620)
Session Type: Abstract Session
Session Time: 3:45PM-4:00PM
Background/Purpose: The American College of Rheumatology recommends HLA-B*58:01 allele testing before the initiation of allopurinol, specifically among Asian and Black/African American patients, due to their increased risk for severe hypersensitivity reactions. However, testing rates remain low at many healthcare facilities. This study aimed to determine whether the introduction of a clinician-facing dashboard, with or without a Best Practice Alert (BPA), would increase HLA-B*58:01 testing rates among eligible patients at two Veterans Health Administration (VA) medical centers.
Methods: In October 2022, we launched a clinician-facing dashboard that displayed HLA-B*58:01 testing results for all patients prescribed allopurinol at two VA Medical Centers. In January 2023, we added a synchronous BPA that appeared at one of the two medical centers whenever a provider entered a prescription for allopurinol. The BPA was presented as blue text on the medication order entry form and stated “HLA-B*58:01 genotyping recommended before rx for Asian or African American.” Using data from the electronic health record, we compared the change in proportion of patients receiving allopurinol prescriptions who had HLA-B*58:01 genotype testing done after implementation of the dashboard (± BPA) at the two VA medical centers.All statistical analyses were conducted using R (R Foundation for Statistical Computing, Vienna, Austria). A p-value of < 0.05 was considered statistically significant.
Results: From October 2022 through December 2023, the number of Asian or African American/Black patients who filled one or more prescriptions for allopurinol was 448 at the BPA + dashboard site and 499 at the dashboard only site. The two sites were similar in complexity, patient volume, location, and academic affiliation (Table 1). Between October 2022 and December 2023, the cumulative percentage of patients who had HLA-B*58:01 testing increased from 13.1% to 36.8% at the dashboard + BPA site and from 1.0% to 3.8% at the dashboard only site (Figure 2A, p< 0.05). Between January and December 2023, the number of Asian or African American/Black patients who had HLA-B*58:01 testing during a single month increased from an average of three per month to ten per month at the dashboard + BPA medical center and from one to three patients per month at the dashboard only medical center (p< 0.05). Allopurinol prescribers at the dashboard + BPA facility were more likely to order at least one HLA-B*58:01 test during the observation period than those at the dashboard only medical center (Figure 2B, 20.3% vs. 10.0%; p=0.003). However, this varied by provider type. Although there was a significant difference in the proportion of non-rheumatologists who ordered genotyping at the dashboard + BPA vs. dashboard alone facilities (15.4% vs. 4.3%; p=0.0004), there was no significant difference in the proportion of rheumatologists who ordered HLA-B*58:01 testing at the two facilities (83.3% vs. 88.9%; p=0.70).
Conclusion: Implementing a dashboard plus simple text BPA was associated with a greater increase in the proportion of patients who completed guideline-recommend HLA-B*58:01 testing compared to implementing a dashboard alone.
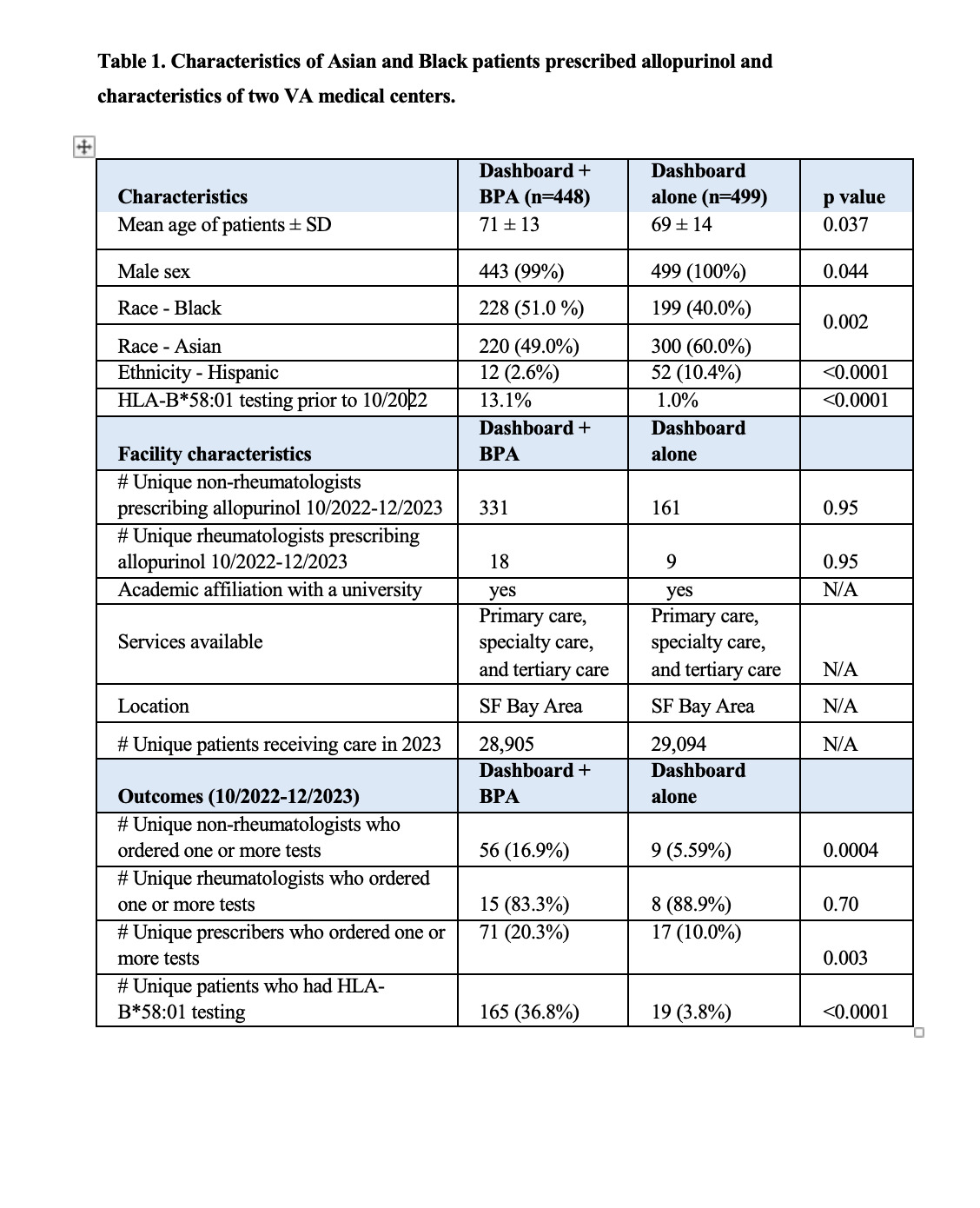 Table 1. Characteristics of Asian and Black patients prescribed allopurinol and characteristics of two VA medical centers.
Table 1. Characteristics of Asian and Black patients prescribed allopurinol and characteristics of two VA medical centers.
.jpg) Figure 1. Interventions: Blue Text alert encountered by providers when entering an order for allopurinol at the BPA+DASHBOARD SITE
Figure 1. Interventions: Blue Text alert encountered by providers when entering an order for allopurinol at the BPA+DASHBOARD SITE
.jpg) 2a. Percent of Asian or African American/Black patients prescribed allopurinol who had HLA-B*58:01 testing
2a. Percent of Asian or African American/Black patients prescribed allopurinol who had HLA-B*58:01 testing
2b. Percent of prescribers who ordered one or more HLA-B*58:01 tests for Asian or African American/Black patients on allopurinol
To cite this abstract in AMA style:
Fadairo-Azinge A, Schmajuk g, Sharma S, Flanagan C, Faghihi-Kashani S, Whooley M. Effect of Implementing a Dashboard with or without a Best Practice Alert on HLA-B*58:01 Testing Rates among Allopurinol Users at VA Medical Centers [abstract]. Arthritis Rheumatol. 2025; 77 (suppl 9). https://acrabstracts.org/abstract/effect-of-implementing-a-dashboard-with-or-without-a-best-practice-alert-on-hla-b5801-testing-rates-among-allopurinol-users-at-va-medical-centers/. Accessed .« Back to ACR Convergence 2025
ACR Meeting Abstracts - https://acrabstracts.org/abstract/effect-of-implementing-a-dashboard-with-or-without-a-best-practice-alert-on-hla-b5801-testing-rates-among-allopurinol-users-at-va-medical-centers/
