Session Information
Date: Tuesday, October 28, 2025
Title: (2470–2503) Systemic Sclerosis & Related Disorders – Clinical Poster III
Session Type: Poster Session C
Session Time: 10:30AM-12:30PM
Background/Purpose: SSc is a rare autoimmune connective tissue disorder; up to 64% of patients with SSc develop interstitial lung disease (ILD), which can result in loss of lung function, diminished quality of life, and premature death. It also contributes a substantial economic burden through healthcare resource use (HCRU), medical expenses, and loss of productivity. This systematic literature review (SLR) evaluated the economic burden and HCRU associated with SSc in patients with ILD (SSc-ILD) and without ILD.
Methods: This SLR (GSK Study 222155) identified eligible studies (patients of any age with SSc, irrespective of severity and subtype) through database searches, with manual searches of recent peer-reviewed SLRs, meta-analyses, and health technology assessment reports. SLR conduct and reporting followed Preferred Reporting Items for Systematic Reviews and Meta-Analyses guidelines. Only cost of illness and HCRU studies published as full text articles, conducted in key countries of interest (United States [US], Canada, United Kingdom, France, Germany, Spain, Japan, and China), with an observation period ending between 2013 and 2023 were selected for data extraction. Cost outcomes included total healthcare, direct, and indirect costs. HCRU outcomes included hospitalization data and length of inpatient (IP) stays. Treatments received for SSc (any use) were also assessed. The Newcastle-Ottawa Scale was used to assess the quality of studies.
Results: Out of 3042 records identified, 54 studies were considered eligible and a total of 17 studies (data reported from US, Canada, England, France, and Spain with sample sizes from 24 to 95,515) were extracted (Figure); all were determined to have an overall low risk of bias.Marked variation in costs associated with SSc overall and SSc with or without ILD were observed across studies (Table 1). Total direct healthcare costs up to $47,095 per patient per year were reported for SSc overall in the US. Where reported, direct healthcare costs were notably higher for SSc-ILD versus SSc without ILD. Patients with SSc-ILD generally had longer IP stays than those without ILD, while data on hospitalization/emergency room visits were limited (Table 2). Treatment rates varied across studies. Glucocorticoids were the most common treatment for SSc/SSc-ILD (global usage: 24.0–74.1%; US rates: 24.0–44.7%), followed by mycophenolate mofetil, while less than 4% of patients received biologics.
Conclusion: These data demonstrate the substantial economic burden and HCRU with SSc and suggest that SSc-ILD has a greater impact on these measures than SSc without ILD, likely due to greater disease severity and associated impairments. However, cost/HCRU estimates varied widely, which may be partially attributable to differences in population criteria and study methodologies. Findings were restricted to five countries reporting scarce indirect cost data. Therefore, there is a need for more comprehensive studies that evaluate a broader range of cost/HCRU outcomes across diverse settings for patients with SSc-ILD, which will help validate and expand upon existing evidence to better characterize the economic burden of SSc-ILD. Funding: GSK
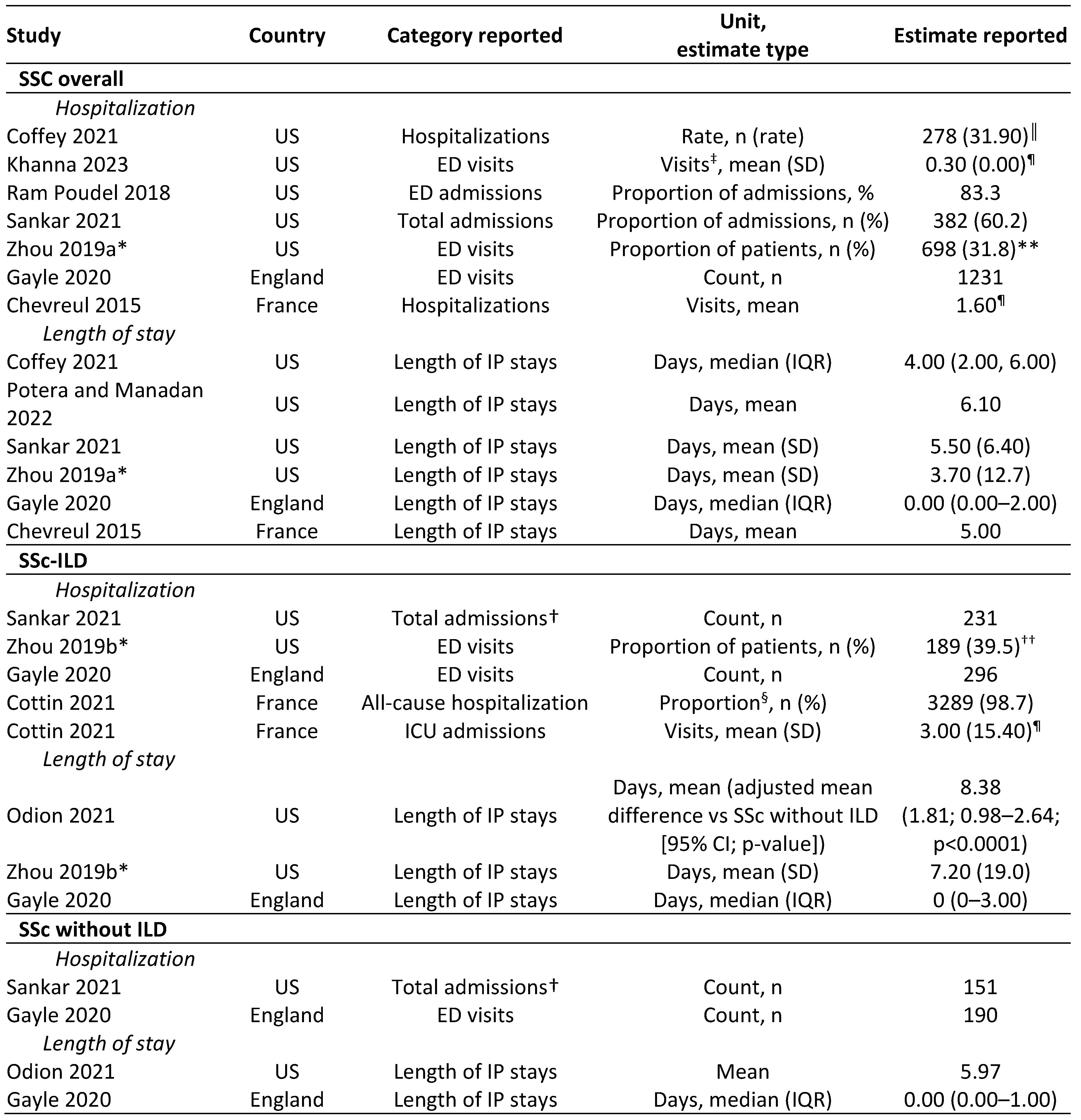 Figure. Flow diagram to summarize identification, screening, inclusion, and extraction of studies.
Figure. Flow diagram to summarize identification, screening, inclusion, and extraction of studies.
*Hand-searching of conference databases included searches of EULAR to identify any additional abstracts of interest not captured by Northern Light; †citation searching included studies identified via reference checks and Google search engine queries.
CCTR, Cochrane Central Register of Controlled Trials; Embase, Excerpta Medica DataBASE; Econlit, Essential Reference Tool for Economic Literature; EULAR, European Alliance of Associations for Rheumatology; HTA, Health Technology Assessment; ISPOR, International Society for Pharmacoeconomics and Outcomes Research; MEDLINE, Medical Literature Analysis and Retrieval System Online; NHS EED, National Health Service Economic Evaluation Databases.
.jpg) Table 1. Summary of cost outcomes (total direct and indirect healthcare costs; studies reporting values per patient per year only).
Table 1. Summary of cost outcomes (total direct and indirect healthcare costs; studies reporting values per patient per year only).
Note that five studies that were suitable for data extraction are excluded from the table due to not reporting total direct/indirect healthcare costs, or per patient per year data. Mean costs are not adjusted for inflation.
*Costs reported after 12-month follow-up; †no studies evaluating SSc without ILD reported total indirect costs or total healthcare costs incorporating direct and indirect; ‡not including medication; §median reported in lieu of mean.
OOP, out of pocket.
.jpg) Table 2. Summary of HCRU outcomes (focus on hospitalization, ED visits, and length of IP stays).
Table 2. Summary of HCRU outcomes (focus on hospitalization, ED visits, and length of IP stays).
Note that two studies that were suitable for data extraction are excluded from the table due to not reporting data on hospitalization or length of IP stays, or for only evaluating incident SSc.
*Data reported after 12-month follow-up; †all scleroderma-related; ‡all services consider individual days, which may include multiple service types; §population comprised patients with at least one visit; estimates reported: ║per 100 person-years; ¶per person per year (where footnotes not included, such estimates were not reported); values at baseline: **0.30 (1.20), ††0.40 (1.00).
CI, confidence interval; ED, emergency department; ICU, intensive care unit; IQR, interquartile range; SD, standard deviation.
To cite this abstract in AMA style:
Glowienka E, Nickolls S, Coady K, Kodi E, Steinfield I, Wilson F, Pepper A, Nihtyanova S, Levy R, Moldaver D. Greater Economic Burden and Healthcare Resource Utilization in Patients with Systemic Sclerosis With Versus Without Interstitial Lung Disease: Results from a Systematic Literature Review [abstract]. Arthritis Rheumatol. 2025; 77 (suppl 9). https://acrabstracts.org/abstract/greater-economic-burden-and-healthcare-resource-utilization-in-patients-with-systemic-sclerosis-with-versus-without-interstitial-lung-disease-results-from-a-systematic-literature-review/. Accessed .« Back to ACR Convergence 2025
ACR Meeting Abstracts - https://acrabstracts.org/abstract/greater-economic-burden-and-healthcare-resource-utilization-in-patients-with-systemic-sclerosis-with-versus-without-interstitial-lung-disease-results-from-a-systematic-literature-review/
