Session Information
Date: Tuesday, October 28, 2025
Title: (2470–2503) Systemic Sclerosis & Related Disorders – Clinical Poster III
Session Type: Poster Session C
Session Time: 10:30AM-12:30PM
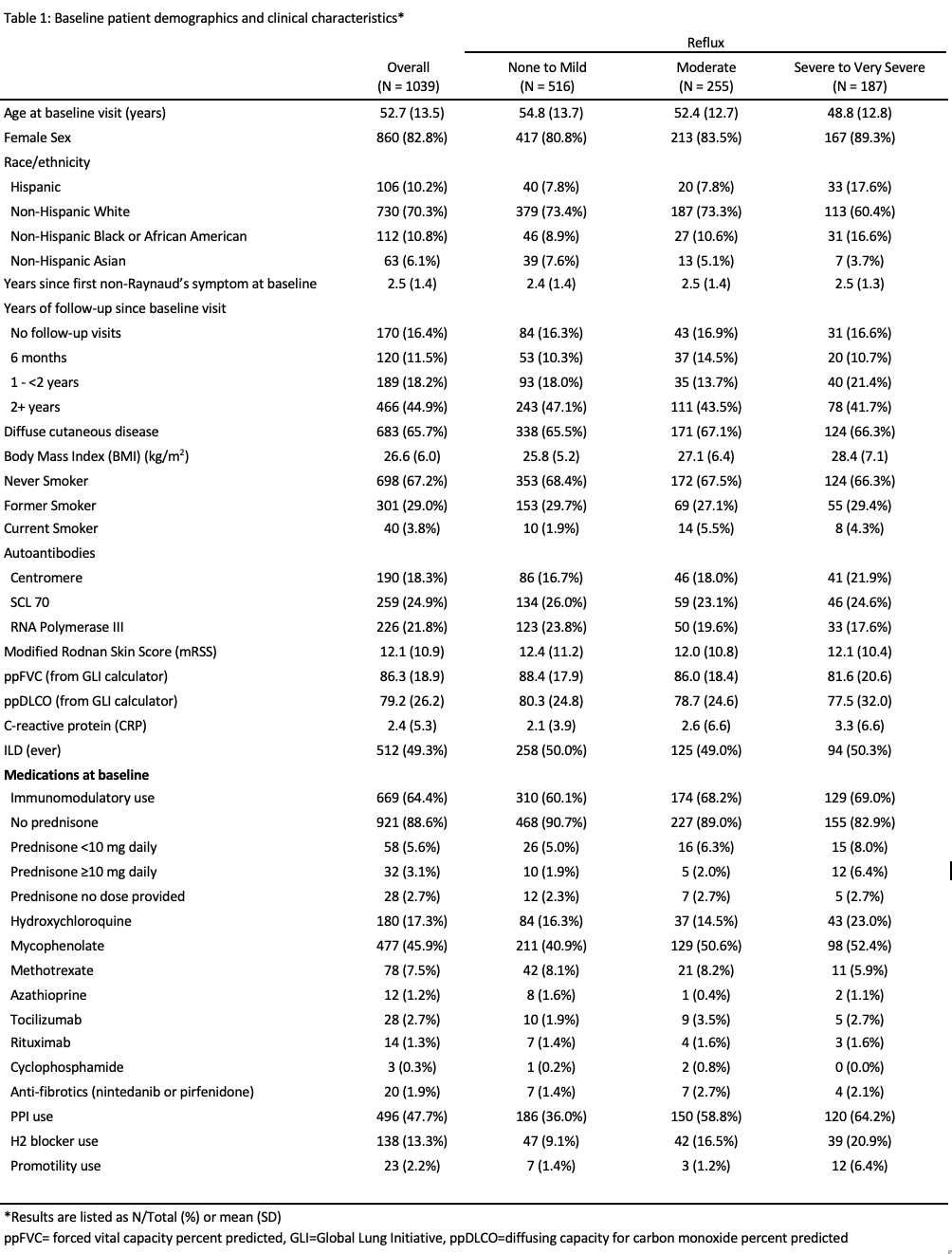
Background/Purpose: Gastroesophageal reflux disease may contribute to the progression of interstitial lung disease in systemic sclerosis (SSc). However, it is unclear whether reflux severity or treatment with proton pump inhibitors (PPIs) impacts longitudinal pulmonary function. The purpose of this study was to examine whether either reflux severity as measured by a validated gastrointestinal symptom questionnaire (UCLA SCTC GIT 2.0) or PPI use is associated with longitudinal pulmonary function in SSc.
Methods: Patients were included if they were enrolled in the CONQUER registry, met 2013 American College of Rheumatology/European League Against Rheumatism (ACR/EULAR) criteria for SSc, completed at least one UCLA SCTC GIT 2.0, and were not pregnant at CONQUER enrollment or during follow-up. We included patients who completed at least 1 pulmonary function test (PFT) for the baseline analysis and at least 2 PFTs for the longitudinal analysis. Baseline missingness and univariate mixed-effect longitudinal regression were used for variable selection to create a mixed-effect longitudinal regression model predicting longitudinal ppFVC using reflux severity on UCLA GIT 2.0 (none or mild, moderate, severe or very severe), PPI use, the interaction between PPI use and GERD severity, as well as appropriate adjustment variables (age, sex, race, disease duration at baseline, follow-up time, baseline modified Rodnan skin score, smoking status, SSc subtype [limited or diffuse], modified Rodnan skin score, scleroderma autoantibodies [Scl70, centromere, RNA Polymerase III, two subserologies, ANA negative, ANA only], and the presence of traumatic ulcers).
Results: One-thousand thirty-nine patients were included, of whom 187 (18.0%) had severe or very severe reflux and 255 (24.5%) had moderate reflux (Table 1). Baseline ppFVC was lower in patients with severe or very severe reflux compared to those with mild or no reflux (81.6% vs. 88.4%). Severe or very severe reflux was significantly associated with longitudinal ppFVC decline in the univariate analysis (-6.1, 95% CI -9.5 to -2.8; p=-0.002; Table 2) but not in the multivariable-adjusted analysis. PPI use was greatest in those with severe-very severe reflux and was independently associated with longitudinal ppFVC decline in both the univariate (-4.9, 95% CI -7.5 to -2.5, p< 0.001) and multivariable-adjusted models (-4.2, 95% CI -7.7 to -0.7; p=0.018). The interaction between PPI use and GERD severity was significant in the unadjusted model (-7.5, 95% CI -11.3, -3.8; p< 0.001) but not in the multivariable-adjusted model.
Conclusion: Severe or very severe reflux was associated with longitudinal ppFVC decline in univariate analysis, but the association was not statistically significant after adjustment for other variables. PPI use was an independent risk factor for ppFVC decline even after adjustment for GERD symptom severity. Use of PPI in patients with SSc may reflect greater esophageal dysmotility or aspiration risk, independent of GERD symptom severity. Alternatively, PPI use may directly contribute to ppFVC decline, possibly by reducing the concentrations and effectiveness of immunosuppressive medications or increasing pulmonary infection risk.
To cite this abstract in AMA style:
Richardson C, Assassi S, Castelino F, Chung L, Evnin L, Frech T, Gordon J, Hant F, Hummers L, Khanna D, Lakin K, Lebiedz-Odrobina D, Luo Y, Makol A, Mayes M, McMahan Z, Molitor J, Moore D, Sandorfi N, Shah A, Shah A, Skaug B, Steen V, Volkmann E, Zahn C, VanBuren J, Bernstein E. GERD Severity, Proton Pump Inhibitor Use, and Longitudinal Forced Vital Capacity in the CONQUER Registry [abstract]. Arthritis Rheumatol. 2025; 77 (suppl 9). https://acrabstracts.org/abstract/gerd-severity-proton-pump-inhibitor-use-and-longitudinal-forced-vital-capacity-in-the-conquer-registry/. Accessed .« Back to ACR Convergence 2025
ACR Meeting Abstracts - https://acrabstracts.org/abstract/gerd-severity-proton-pump-inhibitor-use-and-longitudinal-forced-vital-capacity-in-the-conquer-registry/

.jpg)