Session Information
Date: Tuesday, October 28, 2025
Title: (2437–2469) Systemic Lupus Erythematosus – Treatment Poster III
Session Type: Poster Session C
Session Time: 10:30AM-12:30PM
Background/Purpose: The safety and effectiveness of MMF for the treatment of LN have been tested in adult and pediatric patients. MMF, a prodrug, is rapidly converted to the active moiety mycophenolic acid (MPA) and is recommended as an immunosuppressive medication for active pediatric LN treatment, usually at a dose of 600 mg/m2 body surface area (BSA) twice daily (max 3 gram/day). It has been suggested that an area under the concentration-time curve (AUC0-12h) of MPA at >60-70 mg*h/L is best for reducing proteinuria in LN while levels < 30 mg*h/L are considered ineffective. This is currently being tested in a randomized clinical trial in pediatric LN (PLUMM, NCT05538208). The objectives of this analysis were to evaluate (1) the MPA exposures (AUC0-12h) that are achieved with routine dosing of MMF in pediatric LN patients, and (2) associations of MPA exposures with kidney parameters [serum creatinine, albumin, and protein:creatinine ratio (UPCR)] and prednisone use.
Methods: Pediatric patients with biopsy-proven active LN who participated in the PLUMM trial and were on stable therapeutic doses of MMF underwent abbreviated pharmacokinetic profiling (PK; at trough, 20 minutes, 1 hour, and 3 hours) at study entry, using Volumetric Absorptive Microsampling Combined with Mass Spectrometry (PMID: 40132640). MPA exposure was estimated using an established algorithm (PMID: 26143647) and related to kidney parameters and oral prednisone use.
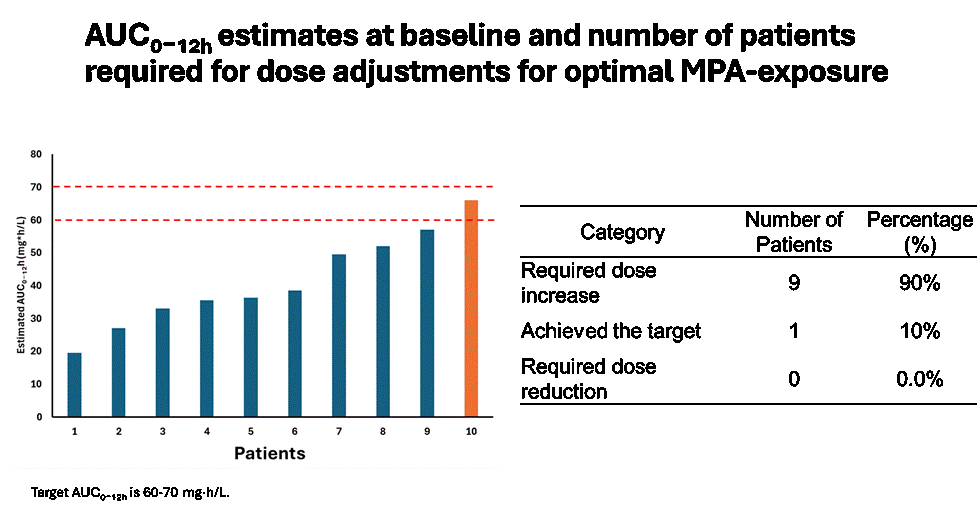
Results: PK profiles were analyzed in 10 enrolled patients receiving stable twice daily doses of MMF for at least 4 days. The median weight and BSA were 56.3 kg and 1.59 m2, respectively, and the median MMF-dose was 2 g/day, resulting in a median MPA-exposure of 37.4 mg*h/L. There were 2 patients achieving ineffective (< 30) MPA exposures and only 1 patient achieved an MPA exposure considered optimal (Figure 1). Serum creatinine, albumin, UPCR, and BSA were all unrelated to MPA exposure. Conversely, there was a moderately strong inverse relationship (r=−0.43) to prednisone dosage (Figure 2). Data from additional patients enrolled in the PLUMM trial will be shown as they become available.
Conclusion: Current BSA-based dosing of MMF seems to generally result in suboptimal MPA exposure, limiting effective reduction of proteinuria in pediatric LN. Our findings confirm that MPA exposure is negatively associated with daily prednisone dosing (PMIDs: 12164891, 21982386). Given the limited sample size, further studies with larger cohorts are necessary. The impact of precision dosing on clinical outcomes will be evaluated after the completion of the PLUMM study.
To cite this abstract in AMA style:
Patel P, Wu E, Cannon L, Hayward K, Okamura D, De Guzman M, Altaffer A, Ardoin S, Cody E, Nadai T, Irie K, Morgan G, Pastrana C, Merritt A, Robben C, Sagcal-Gironella A, Zhao J, Brunner H, Mizuno T. Precision Dosing is Needed to Establish Predictable Exposure to the Active Metabolite of Mycophenolate Mofetil (MMF) in Pediatric Lupus Nephritis (LN) [abstract]. Arthritis Rheumatol. 2025; 77 (suppl 9). https://acrabstracts.org/abstract/precision-dosing-is-needed-to-establish-predictable-exposure-to-the-active-metabolite-of-mycophenolate-mofetil-mmf-in-pediatric-lupus-nephritis-ln/. Accessed .« Back to ACR Convergence 2025
ACR Meeting Abstracts - https://acrabstracts.org/abstract/precision-dosing-is-needed-to-establish-predictable-exposure-to-the-active-metabolite-of-mycophenolate-mofetil-mmf-in-pediatric-lupus-nephritis-ln/

.gif)