Session Information
Date: Tuesday, October 28, 2025
Title: (2377–2436) Systemic Lupus Erythematosus – Diagnosis, Manifestations, & Outcomes Poster III
Session Type: Poster Session C
Session Time: 10:30AM-12:30PM
Background/Purpose: Serum levels of soluble urokinase plasminogen activator receptor (suPAR) have been associated with organ damage accumulation in patients with recent-onset systemic lupus erythematosus (SLE). In this study, we aimed to investigate serum suPAR levels in relation to lupus nephritis (LN), including activity and chronicity features in diagnostic and per-protocol repeat kidney biopsies.
Methods: Serum levels of suPAR from 23 well-characterised patients with LN (of whom 19 were incident cases) from the Cliniques Universitaires Saint-Luc, Brussels, Belgium (Nf21) and the Karolinska University hospital, Stockholm, Sweden (Nf2) were analysed using enzyme-linked immunosorbent assay (suPARnostic® AUTO Flex ELISA, ViroGates, Birkerød, Denmark) at the time of the diagnostic kidney biopsy and prior to commencement of immunosuppressant therapy for active LN, and twelve months after treatment initiation, at the time of a per-protocol repeat kidney biopsy. We also analysed levels of suPAR in serum samples from 15 healthy controls for the purpose of comparisons. The Spearman’s rank correlation coefficient was used to compare levels of suPAR at baseline (BL) and month12 (M12) with serum levels of creatinine, estimated glomerular filtration rate (eGFR), urine protein-creatinine ratio (UPCR), Systemic Lupus Erythematosus Disease Activity Index 2000 (SLEDAI-2K) scores, extra-renal SLEDAI-2K scores (after exclusion of the renal descriptors), and renal histopathological features of activity or chronicity based on the National Institutes of Health (NIH) activity and chronicity index scores, at the same timepoints.
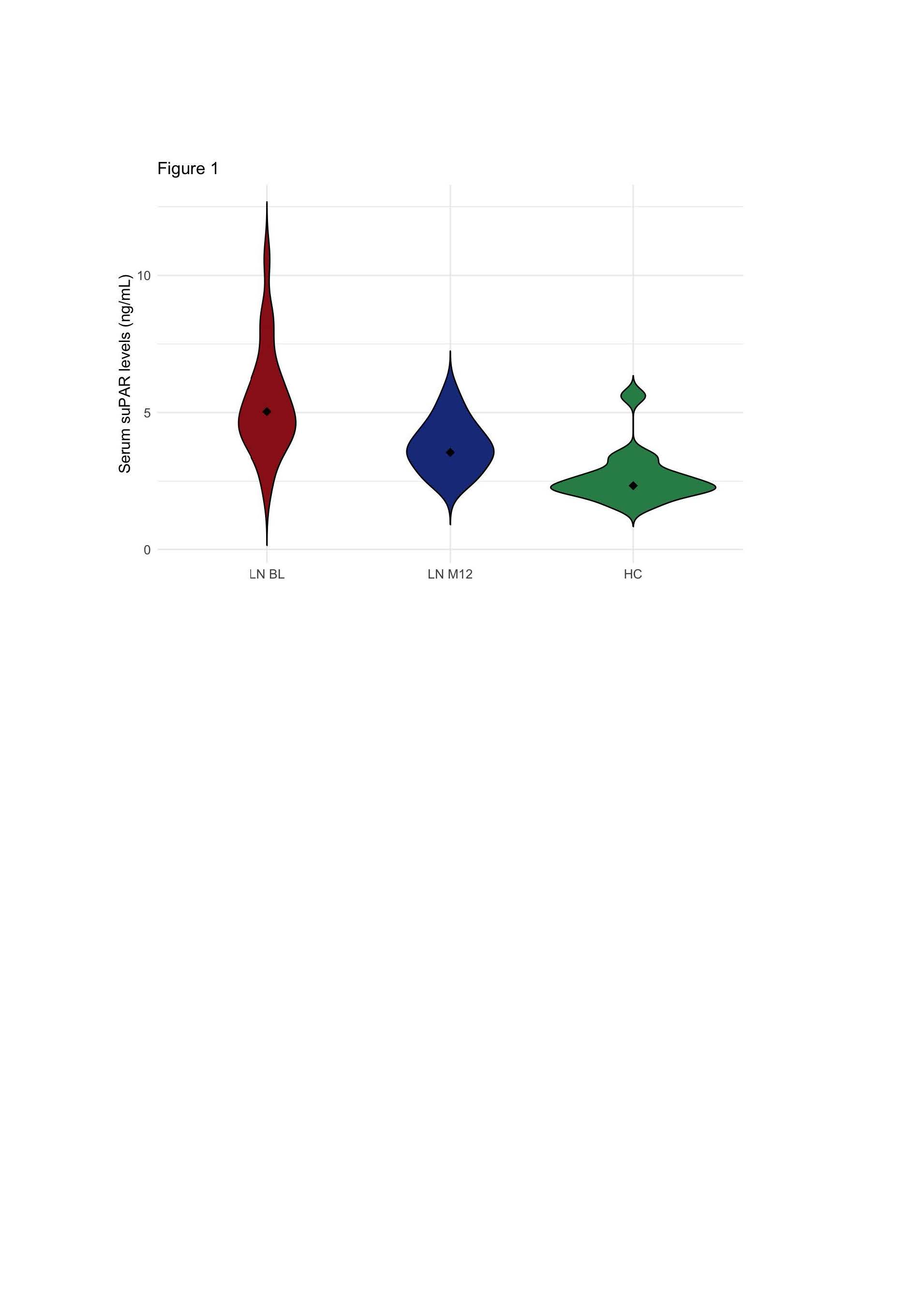
Results: At the time of active LN, patients had significantly higher suPAR levels compared to one year after treatment initiation (mean ± standard error: 5.37 ± 0.40 at BL versus 3.77 ± 0.20 at M12; 95% CI: 0.62–2.56; p=0.002), and compared with healthy controls (2.64 ± 0.25; BL vs HC: p < 0.001; M12 vs HC: p < 0.001; Figure 1). Furthermore, suPAR levels at BL were not correlated with NIH activity index (AI) scores (rho=0.202; p=0.368), but a significant correlation could be seen at M12 (rho=0.595; p=0.007). In addition, suPAR levels were positively correlated with NIH chronicity index (CI) scores at BL (rho=0.541; p=0.008) and the same trend, although not significant, could be seen at M12 (rho=0.440; p=0.066; Figure 2). However, a moderate and statistically significant correlation was seen between baseline suPAR levels and NIH CI scores at M12 (rho=0.554; p=0.017).
Conclusion: In this cohort of LN with repeat kidney biopsies, serum suPAR levels were prominently elevated in patients with active LN compared with healthy individuals and compared to 12 months after initiation of treatment. Levels of suPAR were positively correlated with histopathological activity at month 12 from treatment initiation. An association with chronicity features was also seen at baseline, as well as a correlation between baseline suPAR levels and chronicity features at 12 months. These findings suggest that suPAR levels may prove useful as a biomarker for monitoring LN activity and an indicator of poor prognosis, warranting further investigation.
To cite this abstract in AMA style:
Jagerback S, Houssiau F, Nikolopoulos D, Wirestram L, Lindblom J, Tamirou F, Sjowall C, Parodis I. Associations between Serum Levels of Soluble Urokinase Plasminogen Activator Receptor (suPAR) and Activity and Chronicity Features in Diagnostic and Per-Protocol Repeat Kidney Biopsies in Patients with Lupus Nephritis [abstract]. Arthritis Rheumatol. 2025; 77 (suppl 9). https://acrabstracts.org/abstract/associations-between-serum-levels-of-soluble-urokinase-plasminogen-activator-receptor-supar-and-activity-and-chronicity-features-in-diagnostic-and-per-protocol-repeat-kidney-biopsies-in-patients-wit/. Accessed .« Back to ACR Convergence 2025
ACR Meeting Abstracts - https://acrabstracts.org/abstract/associations-between-serum-levels-of-soluble-urokinase-plasminogen-activator-receptor-supar-and-activity-and-chronicity-features-in-diagnostic-and-per-protocol-repeat-kidney-biopsies-in-patients-wit/

.jpg)