Session Information
Session Type: Poster Session C
Session Time: 10:30AM-12:30PM
Background/Purpose: Disease-modifying antirheumatic drugs (DMARDs) such as methotrexate, sulfasalazine, and leflunomide arecommonly prescribed for RA treatment but carry the risk of leukopenia. The ACKR1 promoter genotype(rs2814778-CC) has been associated with leukopenia and is predominantly present among individuals ofAfrican ancestry. This study aimed to examine whether leukopenia is more frequent among patients with RAwho carried the rs2814778-CC genotype compared to patients without the rs2814778-CC genotype whenusing these DMARDs.
Methods: Retrospective cohort study using the All of Us research program data. We collected genetic and EHR data,including reported race, as well as laboratory data including white blood cell (WBC) and platelet counts afterstarting sulfasalazine, leflunomide, or methotrexate. Leukopenia was defined as a WBC count <3,000cells/mm³, and thrombocytopenia as a platelet count <100,000 cells/mm³. We utilized short-read whole-genome sequencing (srWGS) data from the All of Us Curated Data Release (v7), with direct genotyping of theACKR1 SNP rs2814778 (C/T). We compared patients of EHR reported Black race with the CC genotype to thosewith TT/TC genotypes, as well as to patients of EHR reported White race, because of the known association ofthe CC genotype with lower WBC counts among patients of Black race.
Results: Among RA patients, 463 individuals who were prescribed sulfasalazine, leflunomide, or methotrexate and hadrecorded WBC counts after diagnosis were included in the study. The median age at RA diagnosis was 66 years[IQR: 58.0,73.0], and more than 80% of patients were female. Notably, the rs2814778-CC genotype was absentin the patients of EHR reported White race but found in 68% of the patients of EHR reported Black race. Theoverall leukopenia rate was 36.0% in patients of Black race and 30.9% in patients of White race (p= 0.39).patients of Black race with the CC genotype had the highest leukopenia risk (45.6%), followed by patients ofWhite race with the TT/TC genotypes (30.9%), while patients of Black race with the TT/TC genotypes had thelowest risk (15.6%) (P = 0.01), indicating a significant correlation between genotype and leukopenia risk. Therates of thrombocytopenia were statistically similar acrossall groups (P = 1.00).
Conclusion: Our findings emphasize that individuals of black race with the CC genotype had the highest rates ofleukopenia; highlighting the potential impact of the ACKR1 rs2814778-CC genotype on the risk of leukopeniain patients with RA treated with sulfasalazine, leflunomide, or methotrexate.
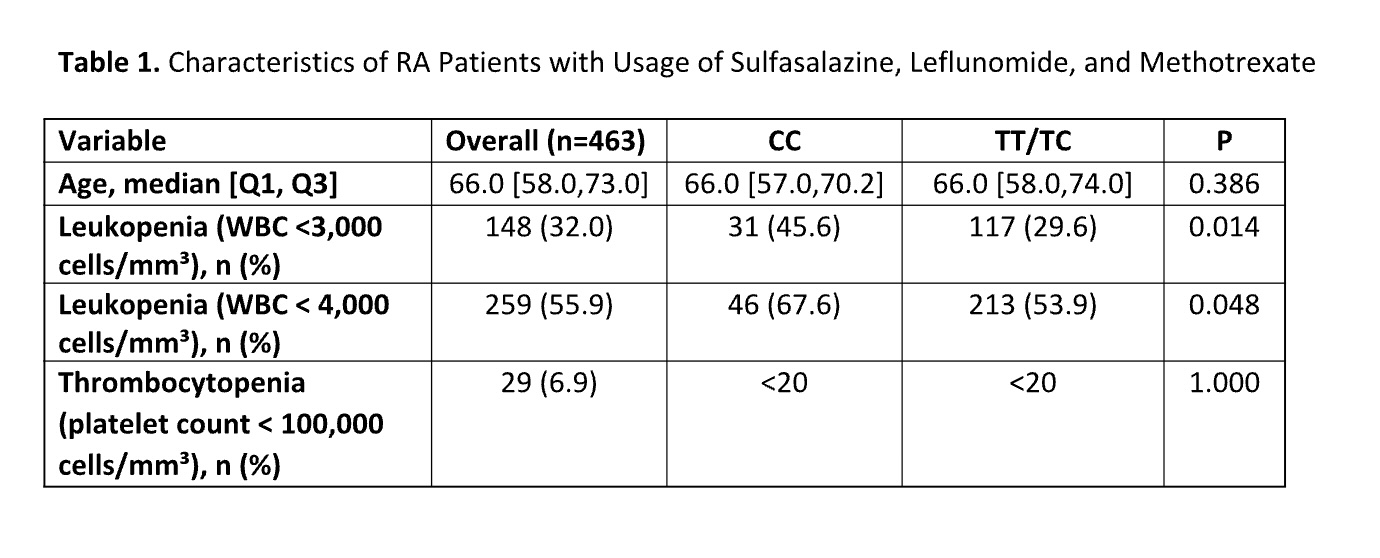 Table 1. Characteristics of RA Patients with Usage of Sulfasalazine, Leflunomide, and Methotrexate
Table 1. Characteristics of RA Patients with Usage of Sulfasalazine, Leflunomide, and Methotrexate
To cite this abstract in AMA style:
Chavez de Paz Solis D, Nepa P, Daniel L, Guo Y, Mosley J, Stein M, Chung C. ACKR1 and Leukopenia in Patients with Rheumatoid Arthritis Treated with Disease Modifying Antirheumatic Drugs [abstract]. Arthritis Rheumatol. 2025; 77 (suppl 9). https://acrabstracts.org/abstract/ackr1-and-leukopenia-in-patients-with-rheumatoid-arthritis-treated-with-disease-modifying-antirheumatic-drugs/. Accessed .« Back to ACR Convergence 2025
ACR Meeting Abstracts - https://acrabstracts.org/abstract/ackr1-and-leukopenia-in-patients-with-rheumatoid-arthritis-treated-with-disease-modifying-antirheumatic-drugs/
