Session Information
Date: Tuesday, October 28, 2025
Title: (2195–2226) Reproductive Issues in Rheumatic Disorders Posters
Session Type: Poster Session C
Session Time: 10:30AM-12:30PM
Background/Purpose: Women with SLE experience higher rates of adverse pregnancy outcomes. Among those with active SLE during pregnancy, lupus nephritis (LN) is associated with particularly poor pregnancy outcomes. Despite the significant impact of LN on pregnancy and offspring, treatment modalities have remained largely unchanged over the past decades. This study aims to utilize data from six international prospective pregnancy registries to describe changes in treatment patterns for active LN during pregnancy over several decades.
Methods: We identified prospective cohorts of pregnancies among women with SLE. We included data from six international prospective registries from the US (North Carolina, Maryland), Italy, Germany, Canada and Israel. Data from each cohort were collected and extracted individually into a harmonized dataset. Active LN was defined as proteinuria scored on the SLEDAI during pregnancy or urine protein–creatinine ratio >500 during any trimester. Pregnancies spanned over three decades (thirty-seven years) and were divided within five time periods. Only pregnancies with active LN, medication data, and known delivery dates were included, allowing for comparison of medication trends over time. Differences in pregnancy outcomes and medication use across time periods were measured using simple statistics.
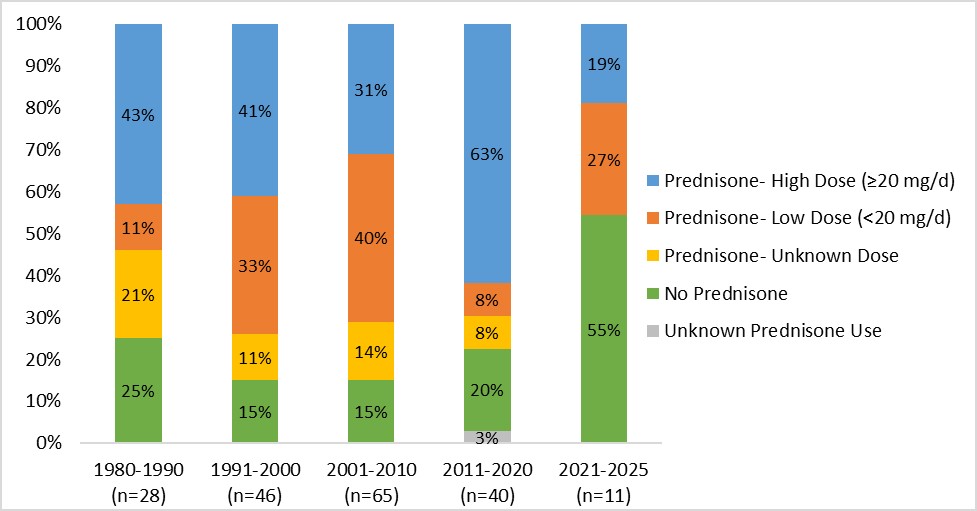
Results: We included 190 pregnancies with active LN during pregnancy. Overall, the average maternal age was 28 years, ranging from 19 to 44 years, and maternal age was unchanged over time (Table 1). Included pregnancies were multiracial, with most identifying as Black (40%). When evaluating pregnancy outcomes, among those with delivery after 20 weeks gestation, 29% developed preeclampsia, ranging from 23% to 44% over time periods. Rates of miscarriage and stillbirth stayed similar across time periods. While overall exposure to prednisone remained relatively stable over time, the percent of active LN pregnancies treated with high-dose prednisone (≥20 mg/day) was variable across time (Figure 1). An increasing trend for both hydroxychloroquine and azathioprine was observed, with hydroxychloroquine increasing from 21% to 100% and azathioprine increasing from 4% to 82% over time. This trend remained similar in analyses stratified by racial groups. Use of other pregnancy compatible DMARDs and/or biologics, including tacrolimus and cyclosporine, are more recent (Figure 2).
Conclusion: Our descriptive study indicates that treatment patterns for active LN during pregnancy across the past three decades have predominately included prednisone, azathioprine and hydroxychloroquine. Despite these treatments, outcomes remain poor, with nearly one-third of pregnancies among women with active LN complicated by preeclampsia. Future research will focus on investigating specific medication dosages and evaluating the treatment effects of azathioprine on adverse pregnancy outcomes, aiming to identify the most effective treatment regimen for women with active LN during pregnancy.
 Maternal characteristics and pregnancy outcomes.
Maternal characteristics and pregnancy outcomes.
.jpg) Pattern of prednisone dosing across time for pregnancies with active LN.
Pattern of prednisone dosing across time for pregnancies with active LN.
.jpg) Use of DMARDs and biologics across time for all pregnancies with active LN.
Use of DMARDs and biologics across time for all pregnancies with active LN.
To cite this abstract in AMA style:
Cintron D, Eudy A, Petri M, Fischer-Betz R, Mockbel A, Nalli C, Andreoli L, Tincani A, Molad Y, Gladman D, Urowitz M, Balevic S, Clowse M. Changes in Treatment Pathways Over Time in Pregnancies with Active Lupus Nephritis [abstract]. Arthritis Rheumatol. 2025; 77 (suppl 9). https://acrabstracts.org/abstract/changes-in-treatment-pathways-over-time-in-pregnancies-with-active-lupus-nephritis/. Accessed .« Back to ACR Convergence 2025
ACR Meeting Abstracts - https://acrabstracts.org/abstract/changes-in-treatment-pathways-over-time-in-pregnancies-with-active-lupus-nephritis/
