Session Information
Session Type: Poster Session C
Session Time: 10:30AM-12:30PM
Background/Purpose: Historically, treatment for systemic juvenile idiopathic arthritis (sJIA) included high dose glucocorticoids (GC) and conventional systemic (cs) disease modifying anti-rheumatic drugs (DMARDs) with significant side effects and poor outcomes. While studies of sJIA patients treated with biologic (b) DMARDs (i.e. IL-1 inhibitors (IL-1i) and IL-6 inhibitors (IL-6i) demonstrated decreased GC use and improved outcomes, less is known regarding longer term outcomes. Using data from the Childhood Arthritis and Rheumatology Research Alliance (CARRA) Registry, we aimed (1) to describe longitudinal trends in medication use over the first 24 months of disease and (2) assess the impact of increased early biologic use on GC use & disease activity (DA) in this observational registry.
Methods: Patients with sJIA enrolled in the CARRA Registry between 2015-2024 were stratified based on disease duration at enrollment. Those enrolled within 60 days of diagnosis and had clinical data at enrollment visit (EV), 6 mo and 12 mo were included in medication and DA analysis. DA assessments included fever, use of systemic GC, and clinical juvenile arthritis disease activity score based on 10 joints (cJADAS10). Chi-Square and Mann–Whitney tests were used to compare the study cohort with all SJIA patients enrolled in the registry. Summary statistics were used to describe medication usage and DA at 6 mo intervals.
Results: Of 920 patients with sJIA, 183 patients (19.9%) met recent diagnosis criteria. Median time from symptom onset to diagnosis was 1 mo (IQR 1-2mo) (Table 1). 87.4% were started on a bDMARD within 60 days of diagnosis (37.7% anakinra and 16.9% canakinumab), and 79.2% were on a bDMARD at some point during their 2 year course (Fig 1). 56.6% of patients who started anakinra switched to canakinumab and 6.5% started on canakinumab switched to anakinra. Of the 12 patients whose first bDMARD was an IL6i, 16.7% switched to an IL1i. bDMARD use decreased over time 79.2% at 12 mo and 18.3% at 24 mo). 14.2% were off all medications by 12 mo and 17.5% by 24 mo (Fig 1). GC use decreased over time, with 64.5% at EV, 30.6% at 6 mo and 13.6% at 12 mo. 14.8% had inactive disease per cJADAS10 ( < 2.5) at EV, with 83.2% achieving inactive disease by 12 mo (Fig 2).
Conclusion: Overall, baseline features of recently diagnosed sJIA patients were not significantly different from other sJIA patients in the Registry. Despite the high incidence of bDMARD use within the first 60 days of diagnosis, nearly half of all patients were exposed to GC early in disease course. The proportion of patients achieving inactive cJADAS10 with or without fever reached 64% and 59.6% respectively by 12mo. At 2 years of follow-up, 17.5% of patients were off all medication.
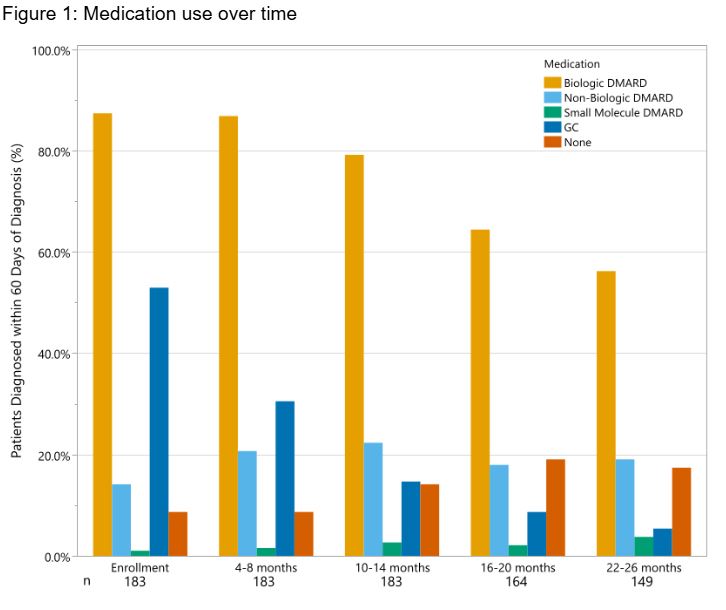 Table 1: Demographic characteristics
Table 1: Demographic characteristics
.jpg) Figure 1: Medication use over time
Figure 1: Medication use over time
To cite this abstract in AMA style:
Gulla C, Son M, Lozy T, Kimura Y, Janow G. Medication Use and Disease Activity in Systemic Juvenile Idiopathic Arthritis in the Childhood Arthritis and Rheumatology Research Alliance Registry [abstract]. Arthritis Rheumatol. 2025; 77 (suppl 9). https://acrabstracts.org/abstract/medication-use-and-disease-activity-in-systemic-juvenile-idiopathic-arthritis-in-the-childhood-arthritis-and-rheumatology-research-alliance-registry/. Accessed .« Back to ACR Convergence 2025
ACR Meeting Abstracts - https://acrabstracts.org/abstract/medication-use-and-disease-activity-in-systemic-juvenile-idiopathic-arthritis-in-the-childhood-arthritis-and-rheumatology-research-alliance-registry/

.jpg)