Session Information
Session Type: Poster Session C
Session Time: 10:30AM-12:30PM
Background/Purpose: Lorecivivint (LOR) is an intra-articular CLK/DYRK inhibitor in clinical development for the treatment of knee OA. A Phase 3 Trial, OA-11 (NCT03928184), assessed clinical and radiographic outcomes over one year following a single LOR injection in advanced knee OA versus placebo (PBO). Patients successfully completing OA-11 were invited to enroll into OA-07 (NCT04520607), a multi-year treatment trial providing annual study treatment injections. The purpose of this analysis was to evaluate OA-11 for any possible differences that could be associated with choosing to enroll into OA-07 or not and thereby impacted the interpretability of the OA-07 results.
Methods: Demographic, outcome and safety data from OA-11 was abstracted by enrollment status into OA-07. Equivalence testing was conducted by enrollment status into OA-07 using two one-sided t-tests. Permutation tests via Monte Carlo simulation (1000 replicated datasets) were also conducted to further assess any predictability in OA-07 enrollment status. Baseline-adjusted ANCOVA was also conducted as a part of each study’s analysis plan.
Results: 501 patients enrolled into OA-11; 276 (55.1%) enrolled into OA-07 (N PBO = 138, N LOR = 138). Overall, the adverse event profile was similar between those subjects that enrolled into OA-07 and those that did not. Age, Body Mass Index, baseline WOMAC Pain and Function, as well as medial joint space width (JSW) were equivalent between those that enrolled into OA-07 and those that did not. Percentages of female, white race, Hispanic/Latino ethnicity, KL Grade 2 and unilateral symptomatic OA were similar between studies but could not be determined as equivalent. WOMAC Pain, WOMAC Function and medial JSW, however, at the end of OA-11 were also equivalent between those that enrolled into OA-07 and those that did not. Permutation tests also indicated that the population enrolled into OA-07 appeared to be a random sample, as all results were stable and not predictive of enrollment status. Statistically significant improvements were observed for LOR vs PBO in WOMAC Pain [0-100] in OA-07 six months after injection (Visit 2E ∆=-4.52, 95% CI [-8.92, -0.13], P=0.044), as well as at twelve months (Visit 3E ∆=-5.19, 95% CI [-9.86, -0.53], P=0.029) (Figure 1). WOMAC Function [0-100] was numerically improved in OA-07 at six months but was significantly improved at twelve months (Visit 3E ∆=-4.86, 95% CI [-9.36, -0.35], P=0.035) (Figure 2). In Year 2 (crossover portion) of OA-07, all PBO patients receiving LOR showed mean improvements in WOMAC Pain and Function from Visit 3E observations.
Conclusion: Examining demographics, outcomes and safety data from the OA-11 trial did not elicit any prediction to patients that enrolled into OA-07 but supports OA-07 as a random sample of the OA-11 population. The impact of COVID on the conduct and results of OA-11 may have relegated the Phase 3 trial into a large run-in, possibly allowing patients to normalize over the course of a year prior to the start of OA-07. In OA-07, improvements in pain and function in LOR compared to PBO were seen at six and twelve months. LOR continues to show promise as a safe, disease-modifying knee OA treatment.
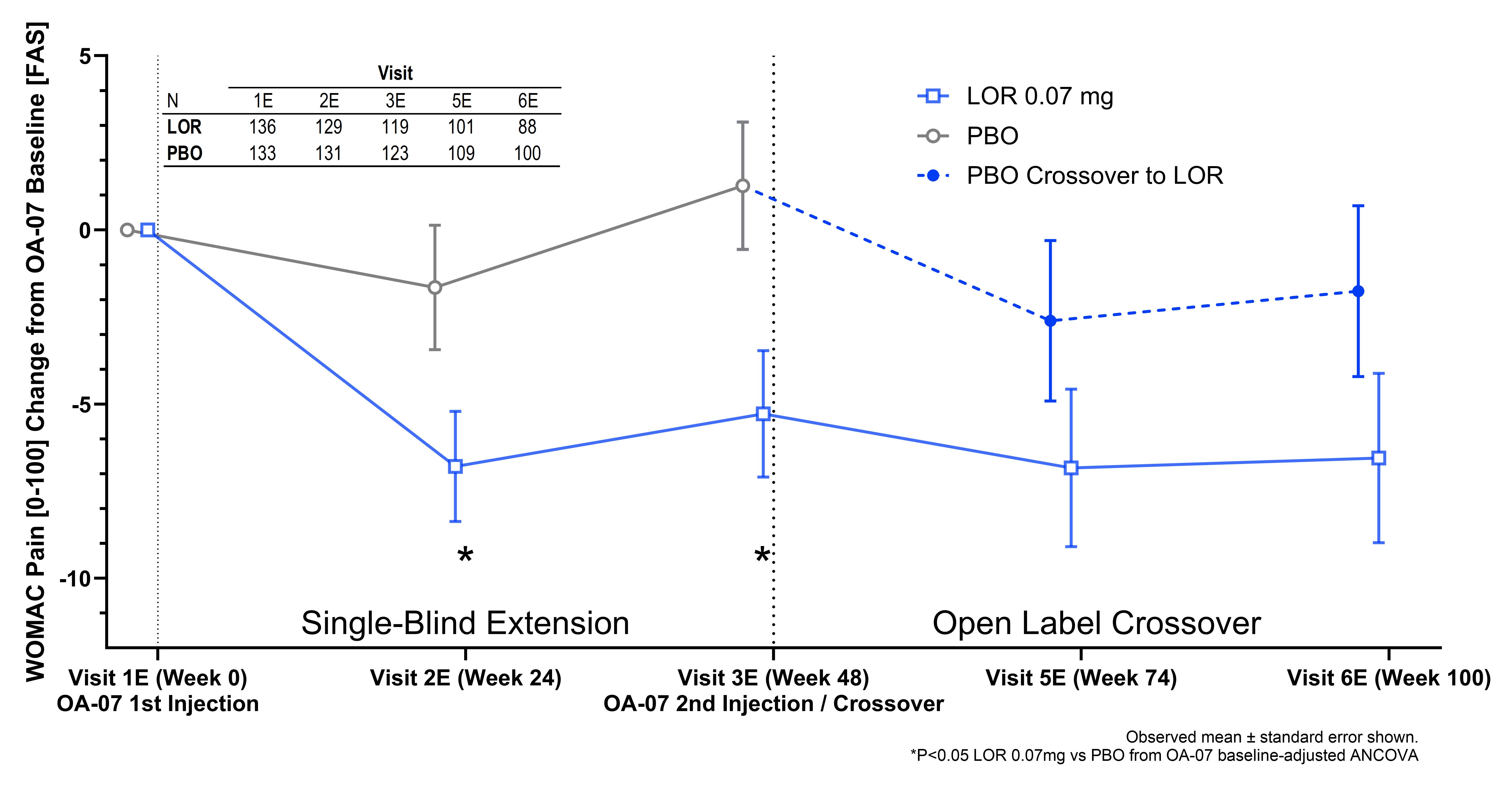 Figure 1. Change from OA-07 Baseline in WOMAC Pain
Figure 1. Change from OA-07 Baseline in WOMAC Pain
.jpg) Figure 2. Change from OA-07 Baseline in WOMAC Function
Figure 2. Change from OA-07 Baseline in WOMAC Function
To cite this abstract in AMA style:
Swearingen C, Yazici Y. Evaluation of a Parent-Trial as a Run-In Period: Efficacy and Safety of Repeat Injections of Lorecivivint over Three Years [abstract]. Arthritis Rheumatol. 2025; 77 (suppl 9). https://acrabstracts.org/abstract/evaluation-of-a-parent-trial-as-a-run-in-period-efficacy-and-safety-of-repeat-injections-of-lorecivivint-over-three-years/. Accessed .« Back to ACR Convergence 2025
ACR Meeting Abstracts - https://acrabstracts.org/abstract/evaluation-of-a-parent-trial-as-a-run-in-period-efficacy-and-safety-of-repeat-injections-of-lorecivivint-over-three-years/
