Session Information
Date: Tuesday, October 28, 2025
Title: (2015–2051) Miscellaneous Rheumatic & Inflammatory Diseases Poster III
Session Type: Poster Session C
Session Time: 10:30AM-12:30PM
Background/Purpose: The potential of B-cell-depleting therapies to diminish humoral responses is recognized, but whether this leads to an increased risk of Coronavirus Disease 2019 (COVID-19) remains unknown. Inebilizumab (INEB) is a humanized, glycoengineered, CD19-directed, monoclonal antibody that depletes B cells, including plasmablasts and some plasma cells, effectively in a targeted manner. MITIGATE (NCT04540497) is an international, randomized, blinded, placebo (PBO)-controlled Phase 3 trial that evaluates the safety and efficacy of INEB as treatment for IgG4 related disease (IgG4-RD). This ad hoc analysis examined whether INEB treatment is associated with an increased risk of COVID-19 infection in adult participants with IgG4-RD.
Methods: Primary results of the MITIGATE trial have been previously reported (Stone et al, 2024). Eligible participants were randomized 1:1 to inebilizumab or placebo and were treated on day 1, day 15, and week 26 of the 1-year randomized controlled period (RCP). Ad hoc analyses were performed to assess the risk of COVID-19 infections with INEB treatment, as COVID-related endpoints were not prespecified. First participant was enrolled October 26, 2020, after COVID-19 was initially reported (December 2019) and before the first vaccine received approval (December 2020). To determine the relationship between COVID-19 infections and immune response, the lowest recorded values of IgG, IgM, and IgA were measured and compared among participants with COVID-19 infections. Vaccination status, incidence of COVID-19, and safety were evaluated.
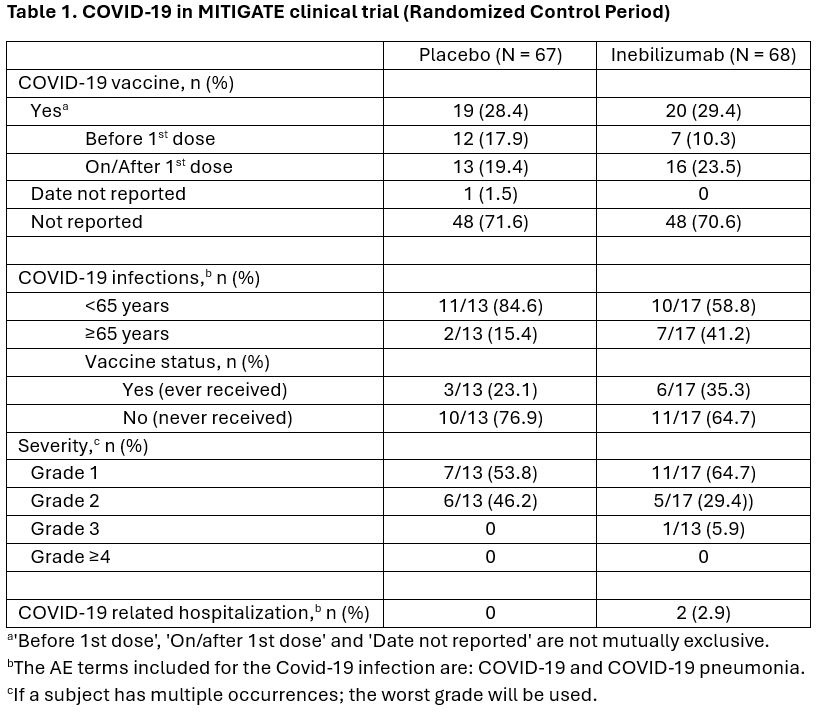
Results: In the trial population, 28.4% (n=19/67) of PBO-treated and 29.4% (n=20/68) of INEB-treated participants had received a COVID-19 vaccination. COVID-19 infection was reported in 13 (19.4%) PBO-treated and in 17 (25.0%) INEB-treated participants. Most infections were in participants < 65 years of age (84.6% PBO, 58.9% INEB). The majority of infections were mild [Grade 1; PBO: 7/13 (53.8%); INEB: 11/17 (64.7%)] or moderate [Grade 2; PBO: 6/13 (46.2%); INEB: 5/17 (29.4%)]. Serious COVID-19 occurred in 0 PBO-treated and 2 INEB-treated participants, both requiring hospitalizations, but were not life-threatening (Table 1). Both of these subjects had been vaccinated for COVID-19 prior to COVID-19 infection. There was no significant association between immunoglobulin reductions and occurrence of serious COVID.
Conclusion: Treatment with INEB was not associated with a higher incidence or severity of COVID-19 compared with placebo. Despite B-cell depletion, infections were mostly mild, and serious infections were infrequent. These findings support the safety of INEB in the context of the COVID-19 pandemic.
To cite this abstract in AMA style:
Khosroshahi A, Culver E, Zhang W, Okazaki K, Tanaka Y, Lohr M, schleinitz n, Dong X, Cheng S, Cimbora D, Stone J. Incidence of Coronavirus Disease 2019 in Participants with IgG4-Related Disease Treated with Inebilizumab in the MITIGATE Study [abstract]. Arthritis Rheumatol. 2025; 77 (suppl 9). https://acrabstracts.org/abstract/incidence-of-coronavirus-disease-2019-in-participants-with-igg4-related-disease-treated-with-inebilizumab-in-the-mitigate-study/. Accessed .« Back to ACR Convergence 2025
ACR Meeting Abstracts - https://acrabstracts.org/abstract/incidence-of-coronavirus-disease-2019-in-participants-with-igg4-related-disease-treated-with-inebilizumab-in-the-mitigate-study/