Session Information
Date: Tuesday, October 28, 2025
Title: (1990–2014) Metabolic & Crystal Arthropathies – Basic & Clinical Science Poster II
Session Type: Poster Session C
Session Time: 10:30AM-12:30PM
Background/Purpose: Among patients (pts) with chronic refractory gout (CRG), traditional urate lowering therapies are often inadequate, necessitating advanced therapies. Pegloticase, a recombinant uricase enzyme, rapidly lowers serum uric acid, reducing flares and tophi in CRG. However, real-world data on its effectiveness, particularly with methotrexate co-treatment, remains limited. This study assessed gout flare outcomes pre and post treatment amongst pts with uncontrolled gout treated with pegloticase.
Methods: The study included adults diagnosed with gout who were newly treated with pegloticase between January 1, 2018, and December 31, 2021 in the MORE2 registry or Medicare Fee-For-Service (FFS) databases. Pts were required to have at least 360 days of baseline and follow-up enrollment from the index date (date of first pegloticase dose) and at least 12 infusions of pegloticase within 180 days from the index date. The primary outcome, gout flare, was identified as follows: 1) having an inpatient visit with a primary diagnosis of gout or 2) having an outpatient or emergency department claim with a diagnosis of gout accompanied by treatment or follow-up visit within a 7-day period, or 3) an outpatient or ED claim with a diagnosis for unspecified joint pain accompanied by a claim for oral/injectable colchicine within 7 days. Gout flare rates as well as secondary outcomes of any glucocorticoid prescription and any opioid prescription were assessed during the following time periods: from 360 days prior to index date to the day prior to the index date (baseline), from end of the first 180 days after index date until the earliest event of death, disenrollment or end of study (12/31/2022) (Follow-up 1), and from date of pegloticase treatment discontinuation to the earliest of death, disenrollment or end of study (12/31/2022): (Post completion). The outcomes were compared between baseline and Follow-up 1 and between baseline and post completion using generalized estimating equations.
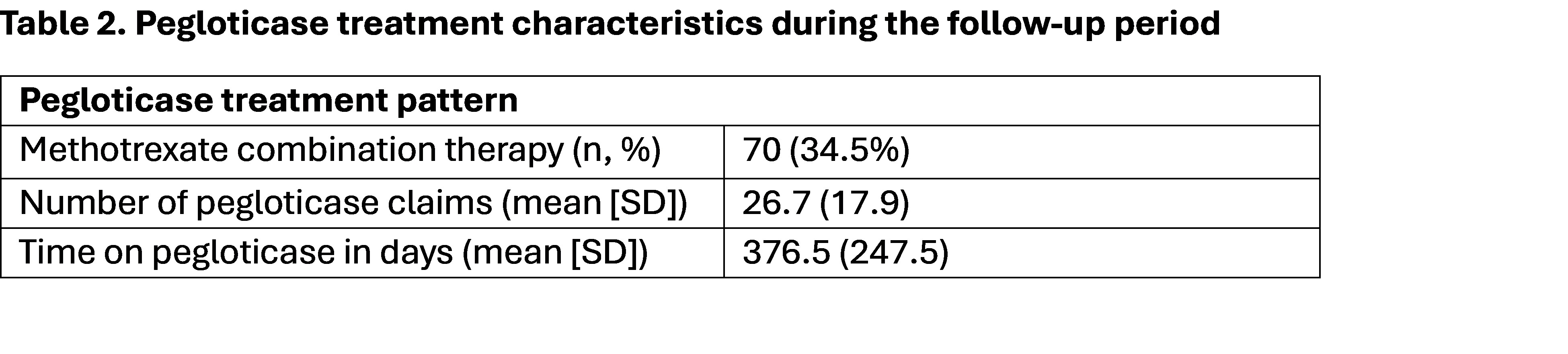
Results: The final cohort included 203 pts initiating pegloticase (mean [SD] age: 67.1[13.1] years; 76.4% male; 67.5% White) with a mean [SD] Elixhauser comorbidity score of 9.0 [9.12] (Table 1). The most prevalent baseline comorbidities were hypertension (85.2%), osteoarthritis (50.2%), chronic kidney disease (57.6%), and type 2 diabetes mellitus (41.87%). The average treatment duration was 376.5 days (SD=247.5), and 70 (34.5%) of the pts had a co-prescription for methotrexate (Table 2). At baseline the cohort experienced a flare rate of 1.3 flares per person years (ppy), which was significantly reduced at Follow-up 1 (0.2 flares ppy) and at post completion period (0.1 flares ppy); (Table 3). Similarly, both proportion of pts with a prescription for glucocorticoids and with a prescription for opioids were lower by at least 20 percentage points during Follow-up 1 compared to baseline and by at least 35 percentage points at post completion compared to baseline.
Conclusion: Pts with CRG, treated with pegloticase in the MORE2 and Medicare FFS, showed a substantial reduction in the rate of gout flares during the follow-up periods. These data further support the use of pegloticase, as a uric acid debulker, for CRG management, thus decreasing the risk of flareup.
To cite this abstract in AMA style:
Buchfuhrer J, Kathe N, Ibiloye E, Kuranz S, Noxon-Wood V, Woods A, Gozalo L. Evaluating Patient Outcomes Pre and Post Pegloticase Initiation among Uncontrolled Gout Patients: Findings from MORE2 Registry and Medicare Fee-For-Service Claims Data [abstract]. Arthritis Rheumatol. 2025; 77 (suppl 9). https://acrabstracts.org/abstract/evaluating-patient-outcomes-pre-and-post-pegloticase-initiation-among-uncontrolled-gout-patients-findings-from-more2-registry-and-medicare-fee-for-service-claims-data/. Accessed .« Back to ACR Convergence 2025
ACR Meeting Abstracts - https://acrabstracts.org/abstract/evaluating-patient-outcomes-pre-and-post-pegloticase-initiation-among-uncontrolled-gout-patients-findings-from-more2-registry-and-medicare-fee-for-service-claims-data/

.jpg)
.jpg)