Session Information
Date: Tuesday, October 28, 2025
Title: (1990–2014) Metabolic & Crystal Arthropathies – Basic & Clinical Science Poster II
Session Type: Poster Session C
Session Time: 10:30AM-12:30PM
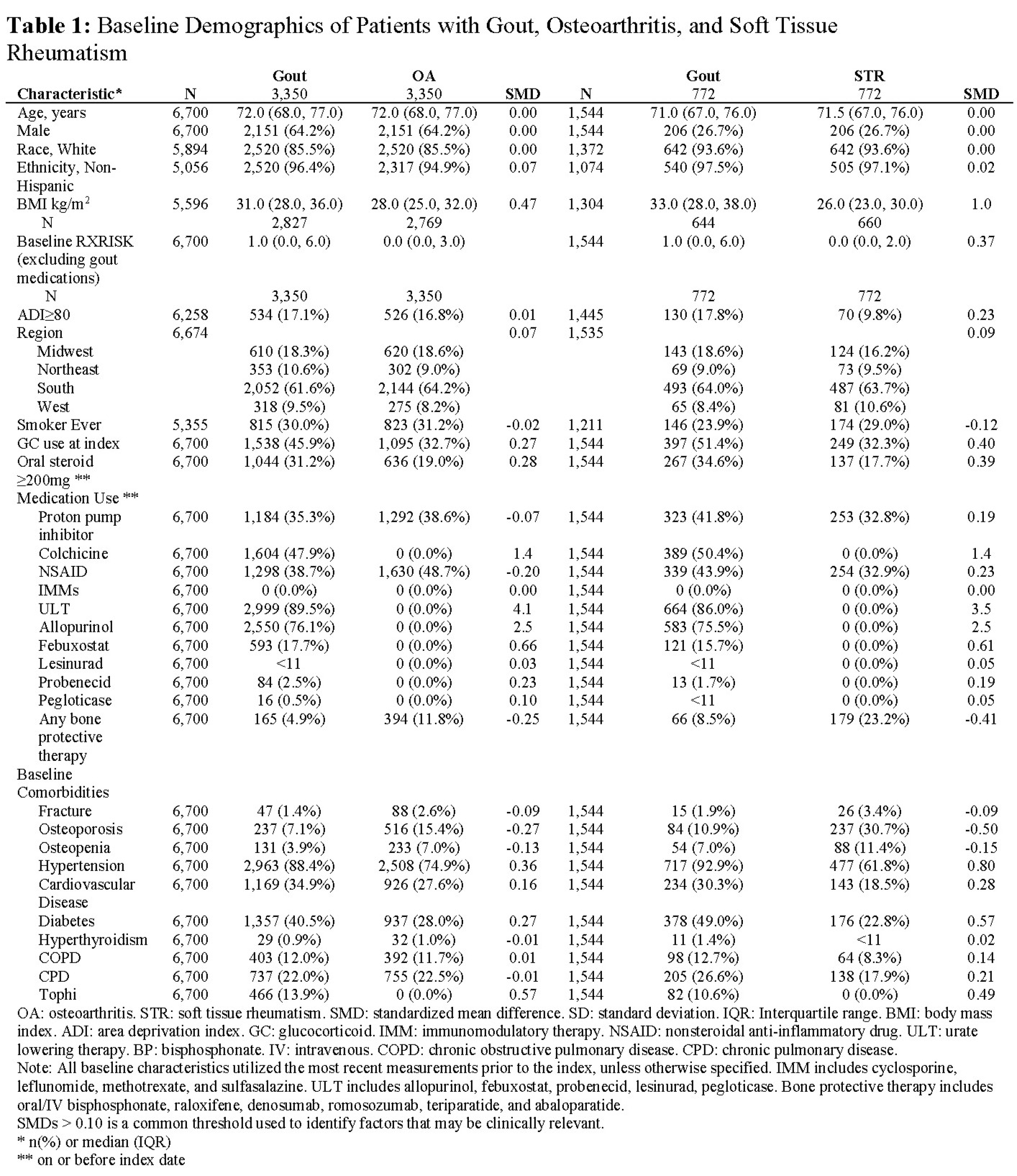
Background/Purpose: Patients with gout may be at high risk for developing osteoporosis and fractures, but osteoporosis may be under-recognized and inadequately managed for gout patients. We evaluated fracture rates and features associated with bone health management in patients with gout and comparator groups using data from the American College of Rheumatology’s (ACR) Rheumatology Informatics System for Effectiveness (RISE) registry linked to Medicare claims data.
Methods: We performed a retrospective analysis of the RISE registry linked to claims data, 2015-2021. Inclusion criteria: age ≥18 at index (later of qualifying cohort diagnosis date, enrollment start+365 days), ≥1 provider visit after index, and no baseline immunomodulators. Gout and two comparator cohorts (osteoarthritis [OA], soft tissue rheumatism [STR]) were identified using ICD-10-CM codes and medications, and patients were selected via this hierarchy. Gout required ≥2 ICD-9/10 codes and urate-lowering therapy or colchicine. Comparator groups required ≥2 ICD-9/10 codes for OA or STR and no ULT or colchicine. Patients in the gout cohort were matched 1:1 by age (±2 years), sex, race, and Medicare enrollment quarter to patients in the OA cohort and (separately) to the STR cohort. We examined medications, comorbidities, demographics, and bone protective therapy use and computed crude and adjusted incidence rates (IRs) and incidence rate ratios (IRRs) for fracture (allowing for multiple events) in the gout vs. comparator cohorts.
Results: We identified 6700 gout patients and matched them to 6700 OA patients. Most were white (85.5%) and male (64.2%), with median age of 72.0 years (Table 1). Approximately 5% of patients with gout used bone protective therapy at baseline, compared to 11.8% in OA. Baseline fracture and osteoporosis diagnosis prevalence was 1.4% and 7.1% in gout vs. 2.6% and 15.4% in OA patients. The gout and STR cohorts were smaller (n=1544 each) and mostly female (73.3%) with demographics otherwise similar to the gout/OA cohorts. Approximately 9.0% of patients with gout used bone protective therapy at baseline, compared to 23.2% in STR. Baseline fracture and osteoporosis diagnosis prevalence was 1.9% vs. 10.9% in gout and 3.4% vs. 30.7% in STR. Excluding the small number of patients using baseline bone protective medications, higher fracture rates were observed in gout patients compared to OA (IRR: 1.4, 95% CI: 1.0,2.0) and STR (IRR: 2.4, 95% CI: 1.0,5.4) (Figures 1 and 2). The IRRs comparing gout to OA patients were minimally different after adjustment for BMI, NSAIDs, diabetes, and comorbidity, but were higher compared to the STR cohort (aIRR 4.1; 95% CI: 1.4,12.4). New bone protective therapies were infrequently used in all cohorts during follow-up (gout: 2.0% vs. OA: 4.5%; gout: 3.4% vs. STR: 10.3%).
Conclusion: Patients with gout experience greater rates of fracture and more commonly have fracture risk factors (e.g. chronic kidney disease, greater glucocorticoid use) compared to OA and STR patients yet are less likely to receive bone protective therapies. Improved awareness of the risk of fracture and osteoporosis in gout appears warranted, as are strategies to protect bone health in gout patients.
To cite this abstract in AMA style:
Holladay E, Woods A, Xie F, Zhang J, Gaffo A, Curtis J, Lamoreaux B. Bone health in patients with gout using real-world U.S. data [abstract]. Arthritis Rheumatol. 2025; 77 (suppl 9). https://acrabstracts.org/abstract/bone-health-in-patients-with-gout-using-real-world-u-s-data/. Accessed .« Back to ACR Convergence 2025
ACR Meeting Abstracts - https://acrabstracts.org/abstract/bone-health-in-patients-with-gout-using-real-world-u-s-data/

.jpg)
.jpg)