Session Information
Date: Tuesday, October 28, 2025
Title: (1972–1989) Measures & Measurement of Healthcare Quality Poster II
Session Type: Poster Session C
Session Time: 10:30AM-12:30PM
Background/Purpose: Children with rheumatic diseases (RD) are at increased risk for developing mental health (MH) issues, including depression, which can negatively impact disease outcomes. MH disorders are underrecognized in pediatric RD. The ACR recently published MH screening guidelines in pediatric RD (Cunningham et al.). The American Board of Pediatrics’ “The Roadmap Project” has a readiness checklist to prepare for outpatient MH screening, which was used to create a depression screening change package with appropriate response documented. An implementation science (IS) framework was applied to gauge the effectiveness of our change package. Goal: To ensure that our change package can be used across diverse pediatric rheumatology clinics. Aims: 1) Assess IS outcomes of change package feasibility and acceptability to aid depression screening with appropriate response. 2) Determine the impact of implementing the change package on contextual factors of relative advantage and adoptability of depression screening.
Methods: Meetings to design the implementation strategy included physicians, psychologists, social workers, parents/patients and an IS scientist. A mixed methods approach using the Health Equity Implementation Framework was designed to implement the change package at 3 pediatric rheumatology clinics to screen patients with RD >8 (PROMIS) or >12 years old (PHQ-9), using a validated depression tool. Each center revised specific quality improvement tools to address unique barriers to screening and responding to screening results. A health equity fishbone diagram captured root cause delinquencies in depression screening. IS outcome and clinical quality measures, along with effectiveness of each change package component, are currently being assessed by the clinical team/parents/patients via REDCap survey at a regular cadence.
Results: We are 5 months into the 12-month implementation strategy. Survey results are reviewed at local and multi-site team meetings. Identified modifications are implemented. At baseline, the 5 parents/patients team members surveyed all agreed with the importance of screening, approved of routine screening, and MH resource pamphlet value. Table 1 is the first staff survey after change package introduction with all 3 teams’ agreement of process feasibility, acceptability and package components’ helpfulness. Initially weekly and then monthly random sampling of patients eligible for screening was done to assess clinical quality measures (Figure 1). This sample of patients were 77% female, 60% White, 29% Black, 15% Hispanic, 93% English primary language, 50% with private insurance, and 41% with Medicaid. The most common RD were JIA and SLE, as expected. Appropriate response to screening employed the change package tools and community/clinic resources (Figure 2). No patient required emergent care.
Conclusion: Using IS, our 3 diverse sites have evaluated a change package that to date is feasible and acceptable, providing a systematic approach to fulfill the pediatric rheumatology community’s desire to screen and further close the gap in addressing mental health. Patients/parents as equal stakeholders helped to identify areas for adjustment and adoptability.
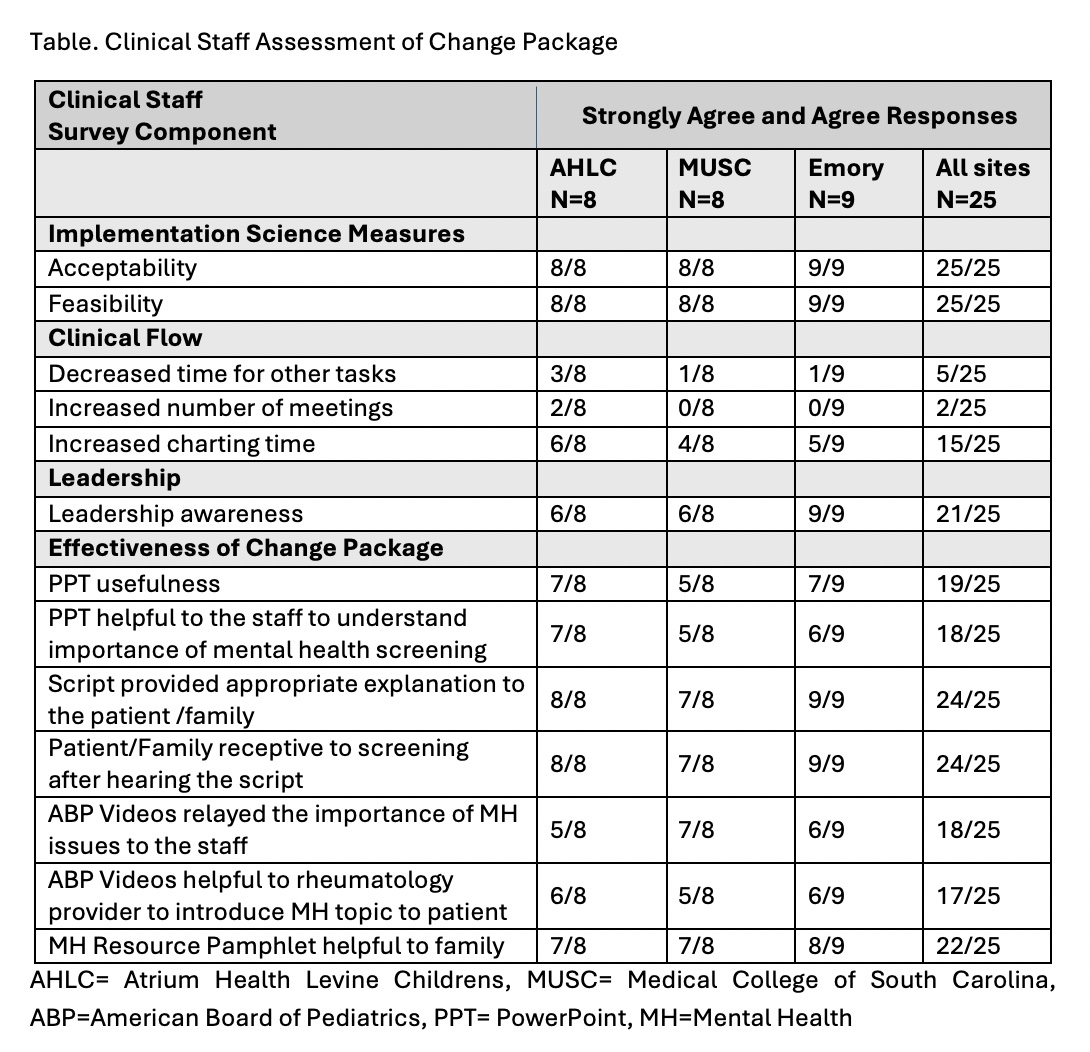 Figure 1. A: 87% of patients were screened for depression. B: 20% of those screened had a positive result. C: for those with a positive depression screen, 36% were mild, 47% moderate, and 17% severe. D: of those with a positive screen, 88% received an appropriate response.
Figure 1. A: 87% of patients were screened for depression. B: 20% of those screened had a positive result. C: for those with a positive depression screen, 36% were mild, 47% moderate, and 17% severe. D: of those with a positive screen, 88% received an appropriate response.
.jpg) Figure 2. In those with a positive depression screen, 88% received one of the responses noted above. Other included situational stressors reviewed with patient and patient to return to office if symptoms persist or worsen (13 of 18), PCP arranging local therapy referral (1 of 18), and corresponded in EMR with Behavioral/Mental Health provider (1 of 18).
Figure 2. In those with a positive depression screen, 88% received one of the responses noted above. Other included situational stressors reviewed with patient and patient to return to office if symptoms persist or worsen (13 of 18), PCP arranging local therapy referral (1 of 18), and corresponded in EMR with Behavioral/Mental Health provider (1 of 18).
Primary Care Provider=PCP, SW=Social Worker, MH=Mental Health
.jpg) Table. Clinical Staff Assessment of Change Package
Table. Clinical Staff Assessment of Change Package
To cite this abstract in AMA style:
Vara E, Gilbert M, Rouster-Stevens K, Buitrago-Mogollon T, Mabus S, Moore J, Vora S. Implementing a Change Package for Depression Screening and Appropriate Response for Children with Rheumatic Disease [abstract]. Arthritis Rheumatol. 2025; 77 (suppl 9). https://acrabstracts.org/abstract/implementing-a-change-package-for-depression-screening-and-appropriate-response-for-children-with-rheumatic-disease/. Accessed .« Back to ACR Convergence 2025
ACR Meeting Abstracts - https://acrabstracts.org/abstract/implementing-a-change-package-for-depression-screening-and-appropriate-response-for-children-with-rheumatic-disease/
