Session Information
Session Type: Poster Session C
Session Time: 10:30AM-12:30PM
Background/Purpose: Older adults with rheumatoid arthritis (RA) treated with biologic disease-modifying antirheumatic drugs (bDMARDs), including anti-TNFs, are at an increased risk of adverse effects. Current guidelines recommend de-escalating DMARDs for patients with low disease activity or remission to optimize the benefit-harm ratio of treatment. We previously found that 20% of older adults with RA de-escalated anti-TNFs. In this follow-up study, we investigated the frequency and factors associated with re-escalation of treatment after de-escalation of anti-TNFs.
Methods: We used 20% Medicare data from 2009-2022 to identify RA patients ≥66 years of age on Part D self-injectable anti-TNF therapy (i.e. Adalimumab, Etanercept, Certolizumab, and Golimumab) with de-escalation by cessation ( > 90-day gap) or by taper ( >50% effective dose reduction), and at least one rheumatologist visit pre- post-de-escalation (i.e. index date). The outcome of interest was re-escalation, defined as restarting the same anti-TNF or another bDMARD after at least a 90-day gap or increasing the effective dose of anti-TNF by >50% after the index date. Information on baseline patient characteristics, concomitant use of conventional synthetic DMARDs (csDMARDs), and changes in average glucocorticoids (GC) dose were collected. The Cox proportional hazard model was used to identify factors associated with re-escalation.
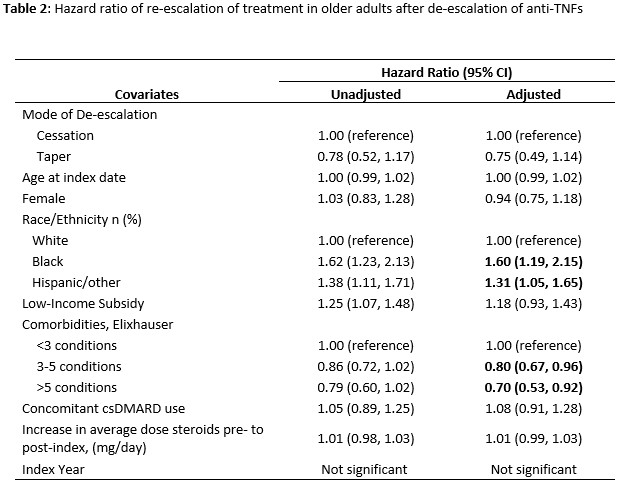
Results: We identified 949 Medicare beneficiaries who met the inclusion criteria. Three in five (61.6%) re-escalated treatment with a median time of 164 (IQR 113-301) days since the index date (Figure 1). Older adults who re-escalate treatment, compared to those with sustained de-escalation, were less frequently non-Hispanic white (72.6% vs 81.3%, p=0.002) and had fewer comorbidities (mean Elixhauser index 2.9 [2.1] vs 3.3 [2.4], p=0.003) (Table 1). The two groups, re-escalators vs. sustained de-escalators, were similar in their age distribution (mean age 74.9 [5.8] vs 75.4 [SD 5.9], p=0.172), female predominance (83.2% vs 81.3%, p=0.447), low-income subsidy (LIS) status (43.9% vs 38.7%, p=0.115), and concomitant use of csDMARDs at index (62.7% vs 61.0%, p=0.590). The change in average daily dose of glucocorticoids pre- and post-index was not significantly different between the two groups (mean 0.6mg/day [4.0] vs 0.5 [SD 3.7], p=0.334). In Cox analyses, re-escalation was more likely in blacks, Hispanics/others, and those with lower comorbidity burdens (Table 2).
Conclusion: Nearly two-thirds of older adults re-escalate treatment within a year of de-escalating anti-TNF therapy, displaying a frequency comparable to that observed in the younger population. These findings suggest that continued exposure to anti-TNF for most older RA patients. In our prior study, de-escalation was associated with a greater comorbidity burden. In this follow-up study, re-escalators had fewer comorbidities, possibly due to concerns for more adverse effects with anti-TNF or other bDMARD therapy. Additional research is needed to identify and understand the subset of older RA patients able to maintain drug-free RA control.
To cite this abstract in AMA style:
Lee J, Martindale J, Makris U, Bynum J. Re-escalation of Treatment in Older Adults with Rheumatoid Arthritis After Anti-TNF Therapy De-escalation [abstract]. Arthritis Rheumatol. 2025; 77 (suppl 9). https://acrabstracts.org/abstract/re-escalation-of-treatment-in-older-adults-with-rheumatoid-arthritis-after-anti-tnf-therapy-de-escalation/. Accessed .« Back to ACR Convergence 2025
ACR Meeting Abstracts - https://acrabstracts.org/abstract/re-escalation-of-treatment-in-older-adults-with-rheumatoid-arthritis-after-anti-tnf-therapy-de-escalation/

.jpg)
.jpg)