Session Information
Session Type: Poster Session C
Session Time: 10:30AM-12:30PM
Background/Purpose: Lupus disproportionately impacts racial and ethnic minorities, yet these groups remain underrepresented in clinical trials. Lupus Therapeutics, overseeing the Lupus Clinical Investigators Network (LuCIN), conducts an annual survey to identify key operational challenges in trial recruitment. In recent years, social media and artificial intelligence (AI) have emerged as potential tools to enhance outreach and streamline operations. However, real-world adoption and effectiveness, especially to engage underrepresented populations, remains uncertain.
Methods: The 2025 LuCIN Annual Survey collected responses from 124 clinical staff across 60 North American sites (Dec 17, 2024, to Feb13, 2025). The survey addressed site use and perceptions of social media and AI tools, recruitment challenges, and preferred strategies to improve inclusion of underrepresented populations.
Results: Of the LuCIN members surveyed, 56% reported having bandwidth to use social media for recruitment, 61% did not find it effective, and 98% require IRB approval to use social media for recruitment. Nearly half (48%) have not used social media to reach the target audience for recruitment in clinical trials, whereas 28% have used social media video content, 27% professional content, and 25% paid advertisements (Table 1). AI adoption was more limited; 34% reported current use in work setting, while others noted policies in development or under review or not at all. Key perceived benefits of AI included translation support (60%), automated chart review (59%), assistance with source documentation (58%) and note-taking (53%). Respondents cited barriers such as institutional restrictions or ethics approvals, lack of trained personnel, and low patient yield (Table 2). 73% cited EDC query resolution challenges and 48% reported burdens associated with manual data transfer from EMRs, highlighting an area for AI to enable efficiencies. Many industry partners include third-party referrals for recruitment which only 16% found effective, supporting the use of alternative strategies. Online educational materials were endorsed by 81% of respondents as the most useful format for engaging patients. 46% prefer a patient navigation program for patients to connect with others in clinical trials. Despite these challenges, sites proposed strategies to advance the recruitment of historically underrepresented individuals relevant to social media including influencer or trusted community leader partnerships, digital campaigns through patient support groups, and educational global marketing campaigns Table 2.
Conclusion: Despite growing awareness of the potential of social media and AI in modernizing lupus trial recruitment, significant barriers limit their application. Widespread adoption is hindered by regulatory barriers, limited institutional support or bandwidth, and concerns about effectiveness and yield. To realize the promise of these tools, especially for engaging underrepresented populations, centralized resources, standardized training, and culturally tailored strategies must be prioritized. These findings support a targeted modernization roadmap for lupus clinical research.
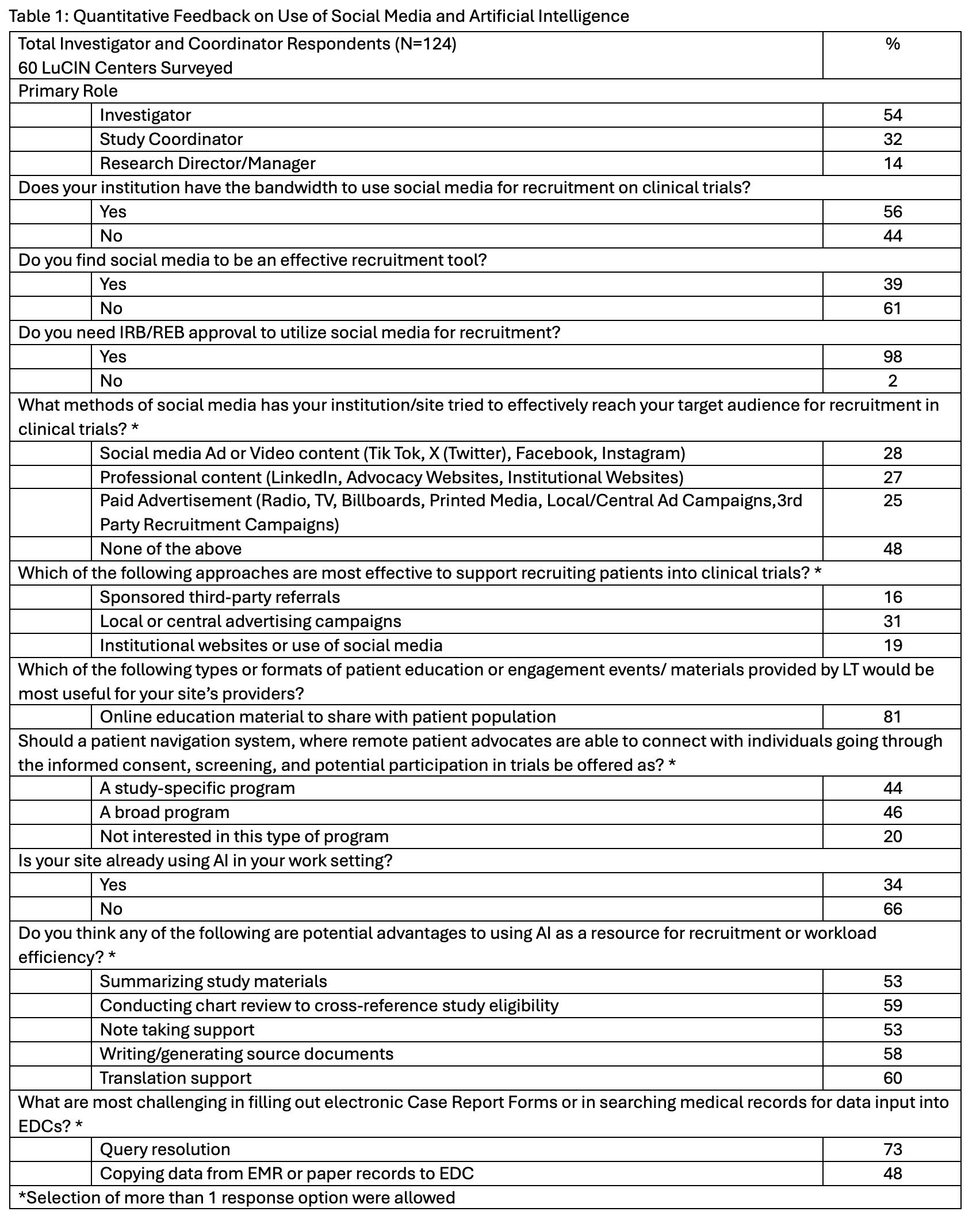 Table 1: Quantitative Feedback on Use of Social Media and Artificial Intelligence
Table 1: Quantitative Feedback on Use of Social Media and Artificial Intelligence
.jpg) Table 2: Qualitative Feedback on Use of Social Media and Artificial Intelligence
Table 2: Qualitative Feedback on Use of Social Media and Artificial Intelligence
To cite this abstract in AMA style:
Jackson B, Caricchio R, Sheikh S, Jolly M, Meriwether J, Irons T, Adjei T, Donovan C, Menezes C, Canton C, Williams A, Merrell M, Bell S. Innovative Clinical Trial Recruitment Approaches in Lupus Research: LuCIN Site Perspectives on Use of Social Media and Artificial Intelligence [abstract]. Arthritis Rheumatol. 2025; 77 (suppl 9). https://acrabstracts.org/abstract/innovative-clinical-trial-recruitment-approaches-in-lupus-research-lucin-site-perspectives-on-use-of-social-media-and-artificial-intelligence/. Accessed .« Back to ACR Convergence 2025
ACR Meeting Abstracts - https://acrabstracts.org/abstract/innovative-clinical-trial-recruitment-approaches-in-lupus-research-lucin-site-perspectives-on-use-of-social-media-and-artificial-intelligence/
